
Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use.
Show details5Evidence on Strategies for Addressing the Opioid Epidemic
Years of sustained, coordinated, and vigilant effort will be required to contain the present opioid epidemic and ameliorate its harmful effects on society. At least 2 million people have an opioid use disorder (OUD) involving prescription opioids, and almost 600,000 have an OUD associated with heroin (HHS, 2016). These numbers are likely to increase in the coming years, regardless of what policies are put in place. Follow-up studies of individuals receiving treatment for OUD involving heroin (e.g., Hser et al., 2001) find very high rates of premature mortality (in the neighborhood of one-third) due to overdose or other complications of the disorder. Thus, even if the nation ramps up treatment availability substantially and immediately, death rates will climb and quality of life will be dramatically reduced for many people for years to come. Likewise, the continued progression of still more people from prescription opioid use to OUD will demand sustained and coordinated effort to establish and implement the scientifically grounded policies and clinical practices necessary to reshape prescribing practices and reduce the occurrence of new cases of prescription opioid-induced OUD.1
What should be done to contain the opioid epidemic and to prevent new cases of iatrogenic addiction and associated overdose, death, and other harms? The purpose of this chapter is to review available evidence on strategies that have been used to address the problems of opioid misuse, OUD, and related deaths. The chapter begins with prefatory sections addressing (1) the nature of the evidence on policies implemented at the jurisdictional level (typically a state or a nation), as opposed to clinical interventions operating at the level of an individual patient; and (2) the need for a systems approach, including the importance of recognizing the potential effects that interventions focused on misuse of prescription opioids have on misuse of opioids more generally. Next the chapter reviews the evidence on the effectiveness of strategies for addressing the opioid epidemic in four categories: (1) restricting supply, such as by regulating the types of products approved for use (e.g., abuse-deterrent opioids) and regulating/restricting conditions of lawful access to approved drugs; (2) influencing prescribing practices, such as through provider education and the issuance of prescribing guidelines; (3) reducing demand, such as by educating patients about opioids and increasing access to treatment for OUD; and (4) reducing harm, such as through provision of naloxone to prevent opioid overdose and needle exchange programs for people who use injection drugs.
NATURE OF THE EVIDENCE
Theoretically, the comparative effectiveness of different opioid-related policies could be quantified through use of randomized controlled trials (RCTs). For example, consider a clinical strategy that eschews prescribing opioids to treat chronic noncancer pain if the patient scores high on a scale used to measure risk of developing opioid addiction. The effectiveness of this strategy for preventing OUD could be evaluated in an RCT in which patients were assigned to either that policy intervention or an alternative one with fewer restrictions on opioid prescription. An RCT is the preferred source of evidence for causal inference because the random assignment is expected to result in comparable groups of individuals assigned to each strategy. In a large RCT of different approaches to opioid prescribing for preventing OUD, for example, one would expect patients in each group to have, on average, the same risk factors for developing OUD. That is, any future differences between the groups in the frequency of OUD could be ascribed to the different treatment strategies to which they were assigned rather than to differences in the characteristics of the individuals receiving each strategy. As a result, the outcome distribution in each group could be interpreted as the counterfactual outcome distribution that would have been observed in that population under the corresponding strategy.2
RCTs, however, are rare for policies that require implementation at the level of an entire jurisdiction, nor are they ethically permissible in many policy contexts. In the absence of RCTs, other sources of evidence are needed to estimate the counterfactual outcome distribution under different strategies. One such source of evidence is the collection of data on individuals who happen to receive the strategies of interest as part of their routine care, often from electronic health records. The so-called observational analyses based on such data are attempts to emulate the RCT that cannot be conducted (the target trial). In these observational analyses, however, the comparability of the groups receiving each strategy is not guaranteed. In the real world, for example, the restricted opioid prescription policy might more likely be applied to individuals visiting providers in urban health care settings who also received other interventions to reduce the risk of addiction. As a result, a direct comparison of the outcome distribution between those who received each strategy would be confounded by the concomitant interventions.
Observational analyses attempt to eliminate bias due to confounding by adjusting for all measured prognostic factors that are distributed differentially between the groups. For example, the comparison might be conducted separately among individuals in urban and rural health care settings. If all confounding factors are appropriately measured and adjusted for, the observational analysis will adequately emulate the target trial and correctly estimate the counterfactual scenarios under each strategy. But even if confounding is eliminated in an observational analysis, this source of evidence is inherently limited with respect to the counterfactual scenarios it can recreate. Analyses of observational data may be helpful for estimating the comparative effects of different treatment strategies applied to a clinical population, but may not capture population-level effects under different policies. For example, an observational analysis of patients of certain health care providers will not quantify effects due to scaling up a treatment strategy as a policy applied to the entire health system.
In fact, this chapter typically investigates the effects of strategies that operate at the level of a jurisdiction, such as a locality or state, or that of the country as a whole. Because random assignment is exceedingly rare in such circumstances (no one, for example, is authorized to randomly assign New Hampshire and 24 other states to receive one policy or to freeze policy in the other 25 states so they can serve well as controls), and observational analyses of clinical populations cannot capture system-wide effects (even if they could successfully adjust for confounding), other approaches are needed. All of these approaches will lack physical randomization of the strategies being examined and therefore will be subject to confounding, but they nonetheless are essential sources of evidence for estimating the effectiveness of various strategies.
Before–After Comparisons
A common nonrandomized source of evidence is before–after comparisons, or the comparison of population outcomes before and after a strategy has been implemented in a single population. Because of underlying trends, however, this comparison may provide a biased estimation of the counterfactual scenarios. For example, the strategy might have been implemented in a population precisely because conditions in that population had been deteriorating. If the underlying factors that gave rise to this trend persisted, conditions might continue to worsen after the strategy was implemented even if the strategy was helpful because it diminished but did not reverse the rate of deterioration. Or the implementation process might move so slowly that the strategy did not take effect until the underlying problem had already exhausted its momentum, and a sort of regression to the mean thus created the illusion that the policy was more effective than it truly was. Therefore, a before–after comparison may not correctly identify the counterfactual of how the world would have looked in the absence of the strategy's implementation.
Ecological Comparisons
Another nonrandomized source of evidence is ecological comparisons, or comparison of outcomes between two different populations, only one of which has received the strategy. Again, however, this comparison may provide a biased estimation of the counterfactual scenarios because the policy may have been implemented in one of the populations precisely because conditions had been deteriorating, or other important between-population differences in prognostic factors may have affected the outcome.
An additional challenge for nonrandomized sources of evidence is that many strategies may exert effects that extend across jurisdictional boundaries or manifest only with a considerable lag. For example, even a successful intervention might noticeably reduce the incidence of overdose only many years after being implemented. Indeed, some interventions that successfully reduced diversion of prescription opioids might, at least in theory, initially increaserather than decrease the number of overdose deaths, even if they reduced deaths in the long run, as the result of an initial surge in deaths among people already addicted to prescription opioids who turned to black market substitutes, whose potency is more variable. Furthermore, some interventions may have different effects depending on the metric employed; thus, for example, distributing naloxone might reduce the number of fatal overdoses but—particularly if there were some risk compensation or other behavioral adaptation—increase the total number of overdose events. Strang and colleagues (1999), for instance, found that 6 percent of individuals in treatment for opioid addiction who were interviewed (9 of 142) reported that access to naloxone might lead them to increase their heroin dosage.
Another problem is that of nonlinear response in systems that have their own internal dynamics. For example, resale or other diversion of prescription opioids by people who had already “traded down” to cheaper black market opioids might cause others to initiate misuse of prescription opioids, others who themselves might later trade down, divert, and supply still others. This problem is illustrated by the difficulty of talking about the number of cases of an infectious disease that are prevented per vaccination as if it were a universal constant, whereas that number in fact depends on the number of other vaccinations being given and the current prevalence of the disease.
THE NEED FOR A SYSTEMS APPROACH
A complementary approach to evaluating intervention strategies implemented at the jurisdictional level in systems with lags and nonlinearities is to use some model of the system in question to project what might be expected with and without the intervention of interest. This approach has been used in a variety of contexts, including air traffic control (Bertsimas and Patterson, 1998; Long et al., 1999; Terrab and Odoni, 1993), fisheries management (Bjørndal et al., 2004; Clark, 1990; Megrey, 1988), vaccination (Goldstein et al., 2005; Kaplan et al., 2002; Medlock and Galvani, 2009), and tobacco control (IOM, 2007, 2015; Levy et al., 2005), among many other important policy domains.
The dynamics of prescription opioid misuse are complicated, particularly when one takes into account the markets for diverted and purely illegal opioids, but a simple sketch helps clarify the value of a systems approach. A typical clinical trajectory that policy changes would like to prevent starts with medically appropriate use of prescription opioids, escalates to misuse and then to OUD, and then evolves to trading down to cheaper black market opioids before manifesting in overdose. Thus, a leaky prescription drug system increases the flow of people into the state of having OUD. People tend to remain in that state for a very long time, an average of 10 to 20 years, with modest flows out of that state through overdose death, death from other causes, or permanent cessation of use.3
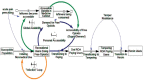
FIGURE 5-1
A systems model of the opioid misuse problem. SOURCE: Reprinted from Wakeland et al., 2015.
The number of overdoses per year might be roughly proportional to the number of people who currently had an active OUD, but this number would not be proportional to the current inflow of new people developing OUD, which is what many interventions aimed at controlling the misuse of prescription opioids would affect most directly. Those interventions would not instantly change the prevalence of OUD and hence would generally not have an immediate effect on overdose. By contrast, interventions that reduced the likelihood that an overdose would occur, or that it would be fatal, might reduce fatalities right away. A fair comparison of the effectiveness of interventions designed to reduce diversion with those designed to reduce the frequency or lethality of overdoses requires a true systems model, not just simple statistics. Wakeland and colleagues (2015) provide an example of such a systems model, reproduced in Figure 5-1.
Constructing such models is a major research endeavor in its own right, and the committee is unaware of any existing model that incorporates all of the strategies discussed in this chapter; therefore, the relative effectiveness of these strategies cannot be compared. Creating such models would have important advantages: it would guide and strengthen surveillance and research, foster a common policy vocabulary among all agencies with decision-making authority over opioid regulation and enforcement (federal, state, and local), and facilitate the exchange of information among them. Investing in research and possible development of such a model is worthy of consideration by the U.S. Food and Drug Administration (FDA) and other agencies. In any event, since no formal systems model now exists, the committee provides an overview of the key conceptual features and implications of a systems approach (without a formal model) to identify some of the considerations that need to be taken into account in reviewing the possible impact of alternative strategies. However, empirical analysis of the various strategies reviewed in this chapter relies on the traditional statistical methods outlined in the previous section.
A Systems Approach to Opioid Misuse
The boundaries delineating governmental agencies' respective responsibilities do not always align with the real boundaries of markets or behaviors concerning OUD and resulting overdose. While the FDA's regulatory authority may give it a particular interest in reducing addiction and mortality caused by prescription opioids, the nation's overall public health interest lies in reducing addiction and mortality caused by opioids of all sorts. A person with prescription opioid–related OUD may escalate his or her opioid misuse, and an overdose leaves a grieving family wondering whether or not the person's last dose was obtained through a prescription.
Prescription and nonprescription opioids intertwine on both the demand and supply sides of the market because all opioids belong to one family of chemicals that operate on similar molecular pathways; the molecules bind to a neuroreceptor regardless of whether they are associated with a prescription. In addition, as shown in Chapter 4, the prescription opioid epidemic is interwoven with the illegal drug market. Therefore, this chapter considers policy options for reducing OUD, mortality due to opioid overdose, and other opioid-related harms among people who have ever used prescription opioids, rather than focusing exclusively on options for reducing misuse of or overdoses from prescription opioids alone.
In the economic sense of the term, all opioids are substitutes (as opposed to complements) in the same sense that oil, gas, coal, nuclear, solar, and hydro are substitute sources of energy for producing electric power. Substitutes are not identical and interchangeable; a molecule of morphine is different from a molecule of fentanyl, just as a barrel of oil differs from a ton of coal. There are distinguishable groupings within broad families of substitutes. Energy policy distinguishes fossil fuels from sources with lower carbon footprints; in this context, one can distinguish partial from complete opioid agonists. But just as one cannot develop a sensible response to global warming by changing only policies toward oil, one cannot develop a sensible response to the nation's opioid problem by adjusting only policies concerning prescription opioids.
The central economic idea about substitutes is that people will tend to use more of item A and less of item B when the price of A falls relative to the price of B, where price is construed broadly to mean the total cost of obtaining and using the item. For opioids, that total cost includes not only the dollar price, but also the time and inconvenience of obtaining the drug and all relevant risks in terms of health and possible criminal justice sanctioning (Moore, 2013; Reuter and Kleiman, 1986; Rocheleau and Boyum, 1994). A related concept is substitution driven by changes in income; as people become poorer, they may substitute hamburger in place of steak and heroin in place of prescription opioids (Petry and Bickel, 1998).
As noted earlier and discussed in greater depth in Chapter 4, in the case of the opioid epidemic, one common pathway to death over the past 20 years has been becoming addicted to prescription opioids, no longer being able to sustain that habit financially, and so trading down to cheaper black market opioids before dying of an overdose or suicide. Trading down can also involve beginning to inject drugs, since that is a more efficient mode of ingesting psychoactive substances. Therefore, additional opioid-relevant public health outcomes include morbidity and mortality stemming from bloodborne infection (e.g., hepatitis C virus [HCV], HIV), both for the individuals injecting and for others (e.g., sexual partners). These outcomes remain relevant even if, for example, no prescription opioids were taken during the month preceding death due to AIDS.
Conversely, finding large amounts of a prescription opioid in the decedent's body does not imply that the person had a prescription. It is common for people who have traded down to black market drugs to retain their prescriptions for purposes of reselling those drugs on the black market. In 2016, typical street prices were $10–$30 for a 30 mg tablet of oxycodone, $5–$20 for a methadone tablet, $3–$8 for Vicodin, and $1 per mcg per hour for fentanyl patches (WSIN, 2016). Thus, diverting to the black market a prescription for two 30 mg tablets per day can produce revenues of $7,300–$21,900 over the course of 1 year. That income is tax-free and mostly pure profit because the copays for those prescriptions are typically small, as is the case for those filled through Medicaid, for example.
Thinking beyond prescription-related misuse becomes all the more important when one recognizes that the same chemicals that appear in prescription drugs are increasingly reaching users not only through diversion but also via distribution chains that are illegal from top to bottom. So even when an autopsy shows that the decedent's body contained a drug that is available by prescription, this does not mean that the fatal dose was obtained through a prescription by the decedent or anyone else.
In particular, drug trafficking organizations increasingly use fentanyl to adulterate black market heroin and counterfeit pills that have been stamped to look like prescription drugs. This black market fentanyl is produced in the same countries—perhaps even in the same laboratories—that sell fentanyl to pharmaceutical companies that supply prescription fentanyl in lozenges and transdermal patches. Likewise, the pill presses and dyes that these firms sell to the drug trafficking organizations that press the powdered fentanyl into counterfeit tablets of opioid painkillers (e.g., oxycodone) and benzodiazepines in North America are the same as those used by other firms to make the tablets sold to the pharmaceutical companies (DEA, 2016a, p. 7). Thus, not only is black market fentanyl the same chemical compound as pharmaceutical fentanyl, but it may even have the same provenance. That in turn means there is no practical way to count precisely how many overdose deaths are due to prescription opioids even in the narrow sense that the proximate cause of death was a dose that had been prescribed.
It is worth noting that black market fentanyl is a relatively recent phenomenon. Until 2014, the number of fentanyl exhibits reported by the National Forensic Laboratory Information System (NFLIS) remained below 1,000, except for a spike to 1,594 in 2006, when a single clandestine lab in Toluca, Mexico, fueled the fentanyl outbreak. The number of exhibits soared in 2014, accompanied by sharp increases in deaths despite no comparable increase in prescribing (Gladden et al., 2016), and reached 13,002 in 2015 (DEA, 2016a).
Price data suggest this trend may continue to intensify. The U.S. Drug Enforcement Administration (DEA) reports that traffickers can buy powdered fentanyl from suppliers for a few thousand dollars per kilogram when buying in bulk (e.g., 20 or 40 kg lots) (DEA, 2016a). Since a counterfeit tablet contains only about 0.9–6.9 mg of fentanyl, the active ingredient can cost high-level traffickers just a penny or two for a pill that wholesales for $6.50 and retails on the street for $10–$20. By comparison, over the past decade, black market retail prices were roughly $500 for a gram of powder 30 percent heroin by weight. So while black market heroin has been much less expensive than (real) diverted prescription opioids, fentanyl is now much less expensive per morphine-equivalent dose than has been the case for black market heroin.
Drug markets are often characterized by substantial price increases as one moves down the distribution chain, but in the case of opioids these increases can be comparatively extreme (in some locations) (Caulkins et al., 2016), which suggests that the current price structure is unstable (Caulkins et al., 2016; Reuter and Kleiman, 1986). The situation is unprecedented, so it is difficult to know how it will develop, but it would not be entirely surprising if the market for counterfeit prescription pills were to undermine the market for real prescription pills. Should this occur, it might reduce the prescription drug overdose problem in its narrowest form, but it would not decrease the total number of opioid-related deaths.
The desire to root opioid policy making in an integrated systems perspective has three corollaries that bear discussion: (1) an ongoing research program is needed to continuously improve understanding of how the various opioids in all their combinations are used and misused in fact, as opposed to just as intended; (2) investment is warranted in an underlying data infrastructure, as opposed to piecemeal efforts local to particular considerations; and (3) the capability to monitor, understand, and model that behavior can be shared among all agencies that have decision-making authority over opioid policy (federal, state, and local), as not all agencies can or should invest in model building within their own silos.
Need for a Formal Quantitative Model
Ideally, an integrated framework for regulatory decision making, discussed further in Chapter 6, would rely on an explicit model of the opioid ecosystem. This is because, as discussed above, decisions made about complex systems with endogenous feedback can be myopic in the absence of a formal model. It would be sensible for the FDA, in collaboration with the U.S. Centers for Disease Control and Prevention (CDC), to commission a panel of experts to develop a quantitative model of prescribed and illicit opioid use and distribution and establish the data infrastructure needed to support and apply that model. With such a model, the FDA and other government agencies could predict the effects of changes in policy or other changes in the opioid ecosystem.
If a model capturing the relevant outcomes in the opioid ecosystem were to be developed, that effort would not be accomplished overnight. The process would take time, and important decisions regarding opioids would have to be made in the interim. For now, then, agencies will need to integrate and weigh data from multiple sources and consider the multiple complex feedback processes without the benefit of a formal model. In Chapter 6, the committee outlines some key attributes of any sound framework for decision making involving opioid regulation. At the very least, these attributes will help in making judgments transparent, highlighting areas of uncertainty and the nature of the qualitative judgments that were made.
In sum, when evaluating past policies and estimating the effects of future interventions, it is necessary to use a comprehensive approach that takes full account of the interactions between prescription and black market opioids. Ideally, this approach could take the form of a quantitative model, although developing such a model would itself be an ambitious research undertaking.
Categorizing Strategies for Addressing the Opioid Epidemic
In traditional policy discourse relating to use of addictive drugs, analysts typically categorize available strategies (including specific policies and interventions) as aiming either to (1) reduce supply or the availability of the addictive drug, (2) reduce demand for the addictive drug, or (3) reduce the likelihood that use of the drug will have harmful consequences (see Box 5-1 for a list of strategies discussed in this chapter). Like all typologies, this one presents challenges of classification, but it will serve well enough in the present context by enabling the committee to summarize the evidence on the effectiveness of the wide range of policies and interventions now being deployed to address the opioid epidemic.
Several preliminary observations are necessary to avoid misunderstanding. First, each strategy has its own costs and entails trade-offs. Obviously, one of the key trade-offs at the heart of this report is the tension between reducing the supply of opioids to reduce harms associated with their misuse and making opioids available to provide pain relief for individuals who have no satisfactory alternative. Second, strategies cannot be fully evaluated in isolation from one another. Sometimes they are seen, mistakenly, to be in tension with one another, as in the example that making naloxone available to prevent a fatal overdose (harm reduction) can counteract policies aiming to discourage opioid misuse. In other cases, different strategies may have additive effects or even potentiate one another, such that each is stronger and more effective than it otherwise would have been; for example, some observers have pointed out that one way in which some tobacco control interventions are effective is through synergy of multiple intervention components (Green and Kreuter, 2010). In still other cases, successful implementation of some strategies (and the effectiveness of a jurisdiction's overall approach) may require that strategies be implemented in tandem with one another. A good example is that a strictly enforced supply reduction strategy may cause substantial harms to individuals with OUD (and to society) unless treatment opportunities are aggressively increased.
Finally, it is important to note that very little research has addressed the relationship among strategies. Thus, strategies A, B, and C may each have a small effect, but what would happen if they were all implemented simultaneously and vigorously is unknown. This limitation is critically important in the context of this report. The data reviewed in this chapter suggest that many strategies might each have a small effect in reducing opioid misuse and related harms, but simultaneous and vigorous implementation of all of these strategies would still leave a huge reservoir of people misusing and addicted to opioids for years if not decades to come.
BOX 5-1
Strategies for Addressing the Opioid Epidemic.
Another important point to make at the outset is that the strategies reviewed in this chapter have been adopted and implemented by a wide variety of public and private entities at the national, state, and local levels. The literature reviewed in this chapter demonstrates that there is currently no national strategy. Nor is there a lead agency responsible for crafting and implementing such a strategy or integrating efforts across levels of government (local, state, or national). While formulating a national strategy and suggesting which agencies should implement it are beyond this committee's charge, this approach is worthy of consideration.
STRATEGIES FOR RESTRICTING SUPPLY
As discussed previously, the responsible clinical use of prescription opioids can be a powerful tool for pain management under some circumstances. The primary area of continuing concern relates to long-term use of opioids to alleviate chronic noncancer pain. A constellation of policies related to lawful access and judicious clinical decision making can help ensure that opioid-related harms are minimized while providing access to these drugs for patients with appropriate clinical indications. This section reviews such supply-side strategies, including regulation of legal access to opioids for legally approved uses. The next section addresses legal regulations and professional policies aimed at reducing lawful access by discouraging unnecessary opioid prescribing or promoting safe prescribing practices. Although both types of strategies aim to control access to opioids, the former focuses on legal restrictions on distribution, while the latter focuses on efforts to influence the decisions of health care providers as the gatekeepers to lawful access by patients.
Regulating the Approved Product: Abuse-Deterrent Opioids as a Case Study
The FDA's decision to approve a new drug follows a rigorous review of product- and indication-specific benefits and risks. In the case of opioids, a drug is reviewed for its ability to provide analgesia, weighed against the potential risk of adverse effects (e.g., dependence, addiction, nausea and other side effects to the patient). Often, the benefit calculus includes product-specific features, such as high-dose extended-release (ER) formulations for pain that is long-lasting and especially severe. The drug is then ultimately approved for use in a specific population for a specific clinical indication, based on the totality of evidence considered by the FDA for that particular population and indication (see Chapter 6 for a suggested approach for FDA decision making on and post-market monitoring of opioids).
However, one consequence of early ER opioid formulations was unexpectedly high misuse. In response, a new product feature—designated abuse-deterrent formulations (ADFs)—has been a focus of FDA policy for addressing the opioid epidemic. ADFs are opioid medications that have been reformulated to reduce the possibility or the likelihood that the medication will be “abused.” While users may misuse opioid medications by swallowing pills whole, the misuse often involves manipulation of the pills. For example, a user may crush the pill and then swallow, snort, or smoke it, or dissolve and inject it. Many ADFs are designed to discourage manipulation either by making the pill difficult to manipulate or by rendering it ineffective or unpleasant once manipulated. Abuse-deterrent technologies include the following (FDA, 2015a):
- Physical designs that are crush/extraction-resistant—For example, OxyContin, a form of ER oxycodone, incorporates a hard polymer matrix that makes crushing or chewing the pill difficult and that transforms into a viscous gel when dissolved in water (which prevents extraction). Formulations that integrate such physical barriers often are referred to as “tamper-resistant opioids.”
- Chemical barriers that prevent extraction of the opioid with solvents.
- Agonist/antagonist combinations that interfere with the euphoria associated with use of opioids—These ADFs include coformulations of opioids with sequestered naltrexone or naloxone. Inadequate pain relief and even acute opioid withdrawal are concerns with the use of these formulations.
- Aversion formulations that include a substance that produces an unpleasant effect if the medication is misused.
- Delivery systems that are resistant to “abuse,” such as subcutaneous implants.
- New molecular entities and prodrugs that have novel effects, such as becoming active only when the pill reaches the gastrointestinal (GI) tract.
- Combinations of these technologies.
The development of ADFs is an evolving area of research, and introduction and regulatory consideration of additional methods are expected.
An industry-sponsored review by Michna and colleagues (2014) found that, relative to placebo, ADFs and non-ADFs were comparably effective and safe for individual patients with noncancer pain. However, it is important to understand that none of the available formulations is designed to prevent all types of misuse—for example, excessive oral ingestion is not prevented by an ADF designed to limit intravenous misuse. Interestingly, currently marketed ADF products do not claim on their labels that they are abuse-deterrent; rather, information on the label describes the studies that suggest abuse deterrence to inform prescribers. The reason is that there is no long-term evidence on the products' real-world impact on reducing misuse, which the FDA would require for such a claim. Indeed, an FDA advisory committee recently voted to remove a particular formulation of oxycodone hydrochloride from the market, citing unexpectedly high potential for intravenous misuse (and associated public health harms) despite attempts to render the drug resistant to insufflation (FDA, 2017a). Thus, while ADFs represent a potentially promising area of opioid drug development, it remains aspirational.
For this reason, the FDA requires that manufacturers of all currently approved ADF products gather data demonstrating the magnitude of the products' effect on real-world misuse relative to existing comparator products and the broader opioid ecosystem (FDA, 2015a). Multiple factors will determine the impact of any given ADF on public health through reduced prescription opioid misuse, addiction, and subsequent misuse of black market opioids. These include prescribing uptake and resulting market share, whether substitutions are made for other comparably harmful prescribed or illicit opioids, and whether ADFs are delivered to those patients with the highest risks of misuse. ADFs may do little to prevent misuse by determined individuals (or actions by a minority of dishonest prescribers), but may play an important role in preventing escalation to misuse. If evidence showed that abuse-deterrent opioids presented truly effective barriers to misuse and that patients with high risk of misuse or diversion were identifiable, one can envision clinical guidelines recommending the prescription of these formulations for such high-risk patients. It remains to be seen whether the FDA's post-market research requirements for opioid manufacturers (see Annex 6-1 in Chapter 6), along with the ADF-specific data gathering mentioned previously, will eventually serve this purpose and reduce the misuse liability of individuals being prescribed opioids.
Another important question is whether the existence of relatively cheap heroin or fentanyl should be taken into account in deciding whether to phase out non-abuse-deterrent opioids, as has been strongly advocated by many analysts. While Severtson and colleagues (2013) report reductions in OxyContin-associated misuse and diversion following introduction of an ADF reformulation, Cicero and colleagues (2012) observe that indicators of fentanyl, hydromorphone, and heroin use went up during roughly the same period. Coplan and colleagues (2013) raise similar concerns based on National Poison System data, as do Cassidy and colleagues (2014) using data on 232,874 individuals assessed for substance use disorder treatment in 2008–2011. Coplan and colleagues (2016) examined the harms associated with reformulated OxyContin compared with other comparator prescription opioids, reporting a noticeable relative decrease for OxyContin, although this study did not specifically examine collateral outcomes such as potential transition to heroin and related harms. A recent state-by-state analysis suggests that the introduction of ADF OxyContin in 2010 resulted in reduced OxyContin misuse, but with a trade-off of increased heroin-related deaths and evidence of an overall trend of increased opioid overdose deaths (Alpert et al., 2017).
Black market exchange could play an additional role for individuals misusing prescription opioids whose access to non-abuse-deterrent formulations was replaced with ADFs. Even if such a person did not know how to defeat the abuse-deterrent technology, he or she could still sell the ADF drugs for cash and use the cash to buy heroin or other black market opioids. ADFs such as the new formulations of OxyContin sell for a moderate discount compared with the non-abuse-deterrent formulations,4 but markets for them nonetheless still exist.
There is also at least the theoretical possibility of “boomerang” effects. Andrew Kolodny, chief medical officer at Phoenix House, has echoed concerns in the field that the abuse-deterrent information on the label might lull some doctors into thinking that these formulations are not misusable and/or are not addictive and so be less cautious in their prescribing (Arlotta, 2016). Also, some attempts to defeat abuse-deterrent properties could create uncertainty as to the actual dose ingested, which might in certain circumstances increase the risk of overdose. Such perverse effects do not necessarily have the potential to outweigh the beneficial effects of ADFs, but that they are readily imagined does underscore the point that no clinical trial finding an ADF to be safe and effective when the unit of analysis is the individual patient necessarily indicates that the ADF will have a net positive effect on public health. In summary, although ADFs of opioids would be expected to reduce some opioid-related harms, it is necessary to consider whether these benefits are offset by their potential effect on movement to illicit markets (either for diverted non-ADF prescription opioids or for illegal drugs such as heroin) among people who misuse opioids or have OUD.
Given the complexity discussed above (and also in Chapter 4), the committee views the evidence surrounding ADFs as not compelling enough to warrant a recommendation at this time. The potential for benefit remains counterbalanced by recent examples of unexpected harm, and ongoing studies will help to clarify the optimal role for ADFs as a strategy for reducing misuse of prescription opioids. The FDA's current cautious approach appears to be well advised. Further discussion of ADFs in the context of the FDA's regulatory oversight of prescription opioids can be found in Chapter 6.
Regulating/Restricting Conditions of Lawful Access to Approved Drugs
Once the FDA has approved an opioid formulation (or other controlled substance) for therapeutic use, federal and state agencies have the authority to control the amount, storage, and distribution of the drug at every stage in the course of commerce. One key purpose of these restrictions is to limit access to and use of the drug to the amounts and indications for which it was lawfully prescribed and to curtail its distribution outside of lawful channels of commerce. This section reviews evidence regarding the effects of the federal and state controlled substances acts and their enforcement on access to approved drugs (i.e., in deterring diversion) and, ultimately, on use (either legal or illegal) of these drugs and associated harms.5 It should be noted that curtailing illegal production and distribution of unapproved/illegal drugs (i.e., heroin and other Schedule I drugs and illegally manufactured versions of legally available drugs) lies outside the scope of this study (see the committee's statement of task in Box 1-1 in Chapter 1). The discussion here also encompasses so-called take-back programs that facilitate the return or destruction of lawfully obtained but unneeded medication, as well as additional state and local restrictions on amounts that can be dispensed or prescribed within specific periods. Related tools include licensing and limiting the class of persons or entities authorized to manufacture, ship, distribute, dispense, and prescribe the approved drugs. The DEA license confers a considerable benefit and provides a source of leverage for regulation and enforcement. Restricting the pool of physicians and other practitioners who are licensed/authorized to prescribe opioids under state or federal law is discussed in the next section. It should be emphasized that all of these efforts to control legitimate access will involve complex policy choices because they may trade off reduced relief from pain and be accompanied by illegal access/use.
“Scheduling” Drugs Under the Controlled Substances Act
In the United States, “controlling” a drug with a “potential for abuse” means placing it within one of the five schedules defined by the Controlled Substances Act (CSA) or shifting it between schedules. (Schedule I is for substances with no “accepted medical use,”6 while Schedules II–V apply to substances with recognized medical value, depending on their potential for abuse. See Chapter 6 for a more specific discussion of the CSA as it relates to opioid regulation.) A moderately large empirical literature exists on the effects of “scheduling” or “rescheduling” a substance under the CSA. This section also refers to studies regarding analogous actions by regulatory authorities in other countries, but the names and particular definitions of the categories differ. Most of these studies are simple “before and after” or interrupted time series comparisons, sometimes with one or multiple outcome indicators (e.g., calls to poison centers).
Scheduling of hydrocodone Perhaps the single most relevant example of opioid rescheduling is the DEA's moving hydrocodone products from Schedule III to Schedule II on October 6, 2014,7 but evidence concerning this event is still emerging. Early studies document clear reductions in prescribing of hydrocodone and increases in prescribing of other opioids, but none examined effects on health outcomes such as death or OUD on the one hand or deficits in pain control on the other.
Oehler and colleagues (2016), for example, document that among emergency department patients in one academic tertiary hospital who received a pain-related prescription, the proportion receiving a prescription for hydrocodone-containing products fell from 58.1 to 13.2 percent following the rescheduling. Seago and colleagues (2016) examined the effects on dispensing by 14 pharmacies in central Texas. They found pronounced reductions in prescriptions for hydrocodone/acetaminophen combinations offset by sharp increases in prescriptions for alternative analgesics, including tramadol and codeine/acetaminophen, leaving total morphine equivalents dispensed after rescheduling only slightly below what they were before rescheduling. The authors conclude that “this study demonstrates several shortcomings of the federal rescheduling of hydrocodone products” (p. 270). However, the ultimate goal of scheduling drugs under the CSA is to reduce misuse and diversion and the addiction, deaths, and other adverse effects associated with misuse. Seago and colleagues do not assess effects on any of those outcomes. Similarly, Haynes and colleagues (2016) report reductions in hydrocodone exposures reported to Texas poison control centers, but increases in mentions of codeine, oxycodone, and tramadol that may reflect substitution. However, this study used no control group, and opioid poisonings may have been increasing for other reasons as well.
Scheduling of other substances in the United States There are other reports of sharp declines in single drug–related indicators after a drug's classification as a controlled substance. Loeffler and Craig (2013) note an 89 percent decline in calls concerning bath salts in the United States after the DEA's October 11, 2011, decision to “control” the substance under the CSA. Likewise, Stogner and colleagues (2012) report that self-reported current and past-year use of salvia fell after Florida classified it as a Schedule I drug on July 1, 2008. Spiller and colleagues' (2010) study of the effects of the scheduling of tramadol by Kentucky and Arkansas is particularly relevant, since it involves an opioid and takes advantage of comparison with two control states (Ohio and West Virginia) that did not schedule the drug. Poison control center cases mentioning tramadol increased in all four states before the scheduling policy intervention, and thereafter continued to increase in the control states but fell in Kentucky and Arkansas.
An older example concerns paregoric. Lerner (1966) documents a geometric rise in the number of paregoric-related arrests in Detroit from 0 in 1955 to 713 in 1963. Michigan ended nonprescription sales of the drug in April 1964, whereupon arrests collapsed, falling to 10 by 1965.
Restrictions on precursor and essential chemicals A related literature explores the effect of adding legal restrictions on precursor and essential chemicals used in the production of controlled substances. McKetin and colleagues (2011)review 10 studies of 13 regulations (plus two enforcement operations) directed at precursors for methamphetamine production in the North American market. Most of these studies found reductions in methamphetamine-related outcomes (of 12 to 77 percent), with no evidence of shifts to other types of drug use; the exceptions were instances in which substitutes for the restricted chemicals were readily available. However, the authors of one of the studies (Dobkin and Nicosia, 2009), while acknowledging short-term effects of that size, stress the impermanence of the reductions as other methods of production were developed over the longer term.
Cunningham and Liu, the lead authors of the majority of the papers reviewed by McKetin and colleagues (2011), also studied regulation of chemicals essential to the production of cocaine. They again report evidence of reductions in various indicators of production and consumption (Cunningham et al., 2015, 2016). In particular, they attribute the dramatic reduction in U.S. cocaine consumption between 2006 and 2010 to regulation of sodium permanganate implemented on December 18, 2006. That decline is significant because it is among the largest in an illegal drug market in recorded history (Caulkins et al., 2014). Thus, key regulatory tools of controlled substance legislation—especially tightening controls (in particular through Schedule II of the CSA) and banning precursor substances to prevent illicit manufacture—can be effective in accomplishing their purposes.
Preventing and Penalizing Diversion of Controlled Drugs
A key element of a regulatory system for controlling dangerous drugs is preventing and penalizing diversion of the drugs from the channels of distribution that have been authorized for medical use. Prescription drugs are diverted to nonmedical use in myriad ways, but it is useful to distinguish three categories: (1) diversion before a prescription has been filled (e.g., theft from production facilities or retail pharmacies), (2) diversion via the filling of a prescription, and (3) diversion after a prescription has been filled.
While the first category undoubtedly occurs, it appears to be of quite modest scale. As noted in Chapter 4, the DEA (2016b, p. 34) reports that in recent years, 12–17 billion dosage units of opioid narcotics were dispensed at the retail level. By contrast, the DEA (2016b, p. 35) reports that in the entire country in 2015, only 9.1 million dosage units were lost to robbery of pharmacies or otherwise “lost in transit.” Those are very small numbers relative to the 12–17 billion dosage units disbursed at the retail level.
By contrast, the third category, diversion after a prescription has been filled, is much more common. One recent survey found that about one in five adults with an opioid prescription self-reported having shared those opioids with another person, most frequently for the purpose of helping to manage pain (Kennedy-Hendricks et al., 2016). However, such individual-level actions generally are not the concern of federal law enforcement, which focuses on misbehavior by DEA registrants and large-scale diversion by industry (Sapienza, 2006).8
Some diversion within the second category, diversion via the filling of a prescription, also falls outside the priorities of federal law enforcement—notably diversion that is driven by the patient (e.g., doctor shopping), facilitated by at most inattention or carelessness by the prescriber but not with criminal intent. The portion of this diversion category that is more likely to attract the attention of federal law enforcement is that which involves the knowing misbehavior of DEA registrants, such as with so-called pill mills.
Some of these actions are civil, not criminal. For example, the DEA has pursued action against CVS in multiple states for filling forged prescriptions or knowingly dispensing to individuals without a legitimate medical need (DOJ, 2016; Wang, 2016). Such action has led to agreements to pay fines in Massachusetts ($3.5 million) and Maryland ($8 million), among other states. The sanction in many DEA cases against practitioners is simply revocation of prescribing privileges, although some of those revocations stem from personal circumstances and errors, such as a practitioner who develops an OUD and is prescribing to him- or herself, not the more egregious cases. The largest criminal case involving prescription drug diversion, Operation Piluted, led to 280 arrests, including 22 doctors and pharmacists, for illegally prescribing and distributing controlled substances, including oxycodone and hydrocodone (DEA, 2015a). One of the doctors charged is accused of selling prescriptions for $500 each, which subsequently yielded profit from sale of the pills on the black market (e.g., selling 100 pills from a prescription at $30 each would gross $3,000).
In a series of investigative journalism stories, The New York Times reporter Katie Thomas (2014a,b, 2015, 2016a,b) documented the criminal activity of InSys Therapeutics. Employees were indicted for offering bribes and kickbacks to doctors and nurses in exchange for their prescribing more of the company's fentanyl product, Subsys, and several of the company's former executives have been charged under the Racketeer Influenced and Corrupt Organizations (RICO) Act. Two doctors who were paid more than $100,000 in “speaking fees” in 2014 were each responsible for prescriptions that generated more than $1 million in Medicare reimbursements.
Drug Take-Back Programs
The DEA, among other agencies and organizations, also tries to reduce the supply of prescription opioids by facilitating the return of unused medications through drug take-back programs. Typically, these are ad hoc or occasional events that allow individuals with unused medications to bring them in to be disposed of properly. Perhaps the best-known is an annual program sponsored by the DEA since 2010 (Stewart et al., 2015).
These programs are popular, and the literature on them is generally favorable, although all but devoid of high-quality evidence concerning effects on final outcomes, such as overdose (Haegerich et al., 2014). Rather, the literature finds that the programs raise awareness (e.g., Yanovitzky, 2016) and that substantial quantities of drugs are brought in for collection (DEA, 2015b; Stewart et al., 2015)—for example, 69.6 million unit doses of medication (of all kinds) brought back in to Operation Medicine Drop in North Carolina (Fleming et al., 2016) over 4 years. However, while the quantities may be substantial in absolute terms, they represent a very small proportion of the total dispensed. Egan and colleagues (2017), for instance, found that over 4 weeks in one community, 21 million units of controlled medication were dispensed, but only 21 thousand were collected.
Furthermore, evaluations of such programs generally cannot assess directly effects on such outcomes as OUD and mortality. Moreover, the reduction in harm may be even smaller than the reduction in volume of medications in circulation if the doses that are voluntarily surrendered are not the ones that would have caused OUD and death had they not been collected. One might speculate that people struggling with OUD or selling pills on the black market would be among those least likely to surrender pills voluntarily.
On the other hand, it is important to note that asking whether take-back programs are an effective way to ameliorate problems with prescription opioids is a very narrow framing. Opioids are one of many categories of medications, and the literature is concerned as much with environmental harms from improper disposal as with harms from nonmedical use.9
Despite the effort invested in occasional take-back programs, proper disposal of unused medications is relatively rare in the United States (Glassmeyer et al., 2009; Law et al., 2015; Maeng et al., 2016), and surveys find that many prescribed drugs are not used (e.g., Kennedy-Hendricks et al., 2016). Maughan and colleagues (2016) found that this was the case for a majority of opioid pills dispensed to patients who had undergone surgical tooth extraction. Likewise, Harris and colleagues (2013) found that one-third of patients prescribed opioids after dermatology surgery did not fill their prescriptions, and 86 percent of those who did had leftover pills. And Welham and colleagues (2015)found that among opioid prescriptions returned for disposal, the majority of the dispensed amount was unused. A large proportion of respondents report keeping medications around, even when they are not needed, and then disposing of them improperly, whether in the trash or down the drain.
Reducing misuse may not be sufficient motivation for members of the public at large to go much out of their way to return drugs; in one study, far fewer participants were motivated by concern about accidental poisoning (14 percent) than by environmental considerations (45 percent) or a simple desire to clean house (68 percent) (Gray and Hagemeier, 2012). The literatures on other environmental problems conclude that getting the public to do what is right (e.g., to recycle) depends on making it very convenient. The United States has largely failed in this regard with respect to disposing of unused medications. Once-per-year take-back programs do not meet that test, and the patchwork of state, local, and pharmacy-specific programs may confuse and deter the public.
By contrast, many peer nations have simple systems whereby most people can return any drug to any pharmacy on any day of the year. Australia's Return Unwanted Medicines program gets high marks in this regard, as do the programs in several of Canada's provinces, including British Columbia's Medications Return Program (Daughton, 2003). Glassmeyer and colleagues (2009) report that many countries in Europe offer a similar service. Sometimes these programs are funded by taxpayers, sometimes by the pharmaceutical industry, and sometimes by a mix of the two. Regardless of who pays, the basic idea of disposing of unwanted materials by operating the standard distribution system backward has many advantages and is a cornerstone of reverse logistics. Box 5-2 provides further detail on one example of a national-level take-back program. It is also important to note that many unused medications are in institutions, such as nursing homes, so ensuring that take-back programs are available to them, not just individual consumers, is important.
Ironically, both environmental and drug control laws make implementing convenient drug take-back programs challenging in the United States (Glassmeyer, 2009). The Resource Conservation and Recovery Act exempts household hazardous wastes from many regulations, but when they are collected, they are regulated. So it is perfectly legal for 1,000 individual consumers to dispose of their unused drugs in the worst possible manner, but if an organization collects those unused drugs and disposes of them in a much better but not ideal way, the organization performing that service may run afoul of the law.
BOX 5-2
An Example of a National Drug Take-Back Program: France's Cyclamed.
Historically, an even greater problem was a requirement of the CSA that scheduled drugs be under the control of law enforcement. Thus, a pharmacy could run afoul of the CSA if it allowed consumers to bring back opioids at any time unless law enforcement personnel were present (Glassmeyer et al., 2009). On September 9, 2014, the DEA published new guidelines allowing certain DEA registrants to become authorized collectors of returned controlled medications (DEA, 2014), although it is unclear whether full advantage is being taken of that new flexibility.
Certainly some organizations find ways to overcome the obstacles and create permanent drop-box options (e.g., Gray et al., 2015), and the committee is not expert in either the legal challenges or logistical practicalities of such programs. However, the advantages of allowing consumers to return medications on any day of the year to any of many locations they visit regularly (e.g., all pharmacies) are clear. As one example of early success, a U.S. pharmacy chain reports that the first year of a program establishing secure dropboxes for unwanted medication (in 600 of its pharmacies across 44 states) has resulted in the collection of 72 tons of medication (Walgreens, 2017).
Education for patients as to why safe disposal is important also is needed. Kennedy-Hendricks and colleagues (2016)report that almost half of survey respondents who were prescribed opioids said they did not recall receiving any instructions regarding safe storage or disposal.
The available evidence suggests that drug take-back programs in the United States can increase awareness about the safe disposal or return of many unused drugs, but effects of these programs on such downstream outcomes as diversion and overdose are unknown. As noted, moreover, many drug take-back programs in the United States are once-per-year events, and the patchwork of state, local, and pharmacy-specific programs may confuse the public. Nevertheless, international examples and the recent success of a year-round disposal program at one pharmacy chain support policies expanding such programs to reduce the amount of unused opioids in the community. The committee recommends that states convene a public–private partnership to implement drug take-back programs allowing individuals to return drugs to any pharmacy on any day of the year, rather than relying on occasional take-back events (Recommendation 5-1).
State and Local Policies Restricting Access
States vary widely in rates of prescribing opioids (e.g., Zerzan et al., 2006), and not surprisingly, evidence indicates that such policy interventions as mandating coverage and reimbursement can affect prescribing of pharmaceuticals generally (Green et al., 2010). There is, after all, a long history of published concern that misinformed and exaggerated fears about liability related to misuse of and addiction to opioids lead regulators to stifle the prescribing of these medications for patients who need them for pain relief (e.g., Hill, 1996). What is less clear is whether one can infer from the variation among states or other evidence whether particular state policies are effective at reducing diversion and misuse of opioids without adversely impacting their availability for pain control. Meara and colleagues (2016), for example, find no association over a 7-year period between opioid-related outcomes in Medicare administrative data and states' adoption of controlled substance laws of the sort described further below.
Haegerich and colleagues (2014) provide a useful review of English-language MEDLINE articles in this literature. Unfortunately, they conclude that the available empirical studies are generally of low quality, and that the outcomes studied are often intermediate, such as prescribing practices, and not final, such as overdose. The largest number of studies uncovered pertained to prescription drug monitoring programs (PDMPs), naloxone, and clinical guidelines, all of which are addressed separately in this chapter; the others are briefly discussed here.
Haegerich and colleagues describe the literature evaluating state policy actions pertaining to regulation of pain clinics (which when they are sources of large numbers of prescriptions may be referred to as “pill mills”) and doctor shopping as “extremely limited” (Haegerich et al., 2014). The pain clinic laws coincide with reductions in the number of clinics and the supply of drugs, but the nature of the evidence is weak. Florida is a special case, discussed further below. Studies of doctor shopping interventions are no better in terms of enabling causal inference concerning health outcomes.
One might say the literature documents that these policies exist and have been implemented, and in a dog-not-barking sense, infer that they can be implemented without resulting in obvious catastrophic failures. Furthermore, there are clear logic models for why one might expect these policies to have some beneficial effect. However, these studies are unconvincing if one adheres to the standards of scientific skepticism and disbelieves that interventions have any bottom-line effect unless clear evidence from high-quality empirical studies demonstrates this to be the case. A Maine law that went into effect January 1, 2017, for example, limits prescriptions for opioids or opioid-containing medications to 100 morphine milligram equivalents (MME) per day. In addition, the law limits the number of opioid pills that can be prescribed to patients (except in cases of inpatient, cancer-related, palliative, and end-of-life care, as well as treatment for substance use disorder) to no more than a 7- and 30-day supply for acute and chronic pain, respectively (Traynor, 2016). In Massachusetts, a new law places a 7-day supply limit on first-time opioid prescriptions for adults and a 7-day limit at any time for minors.10 Yet it remains to be seen what impact these types of restrictions will have on curbing opioid-related harms, particularly for individuals that do not have OUD.
One particular case study merits discussion: Florida's experience circa 2010–2012. Multiple policy interventions were being implemented simultaneously at that time, so it is impossible to use this case study as evidence concerning any one of them. Nonetheless, the changes in adverse outcomes were so abrupt both in absolute terms and relative to other states that it appears highly plausible that some combination of those interventions was responsible for the changes, and hence for averting thousands of premature deaths (Chang et al., 2016; Gau and Brooke, 2016; Johnson et al., 2014; Meinhofer, 2016; Rutkow et al., 2015; Surratt et al., 2014). The interventions were predominantly on the supply side, including closing approximately 600 pain clinics, revoking medical licenses and/or DEA certificates of registration, and placing restrictions on physicians dispensing (as opposed to prescribing) Schedule II–IV controlled substances.11 A PDMP was implemented about 1 year later. The law enforcement component (“Operation Pill Nation”) was led by the DEA but heavily involved state and local law enforcement as well, and targeted not only providers, pain clinics, and pharmacies but also four wholesale distributors.
Meinhofer (2016) shows that these supply reduction measures more than tripled street prices for oxycodone and sharply reduced oxycodone-related mortality and hospitalization with apparently minimal spillover effects on other states, suppliers, or drugs—the only exception being some substitution of heroin, which was small relative to the reductions in oxycodone use. She observes that in the years preceding the operation, 2007–2010, Florida's oxycodone supply per capita had risen from close to the national average to quadruple the national average. After the intervention, it fell back to the national average. Consumption of various substitutes never departed appreciably from national averages, and no other state experienced a spike in oxycodone supply even close to the same magnitude as that experienced in Florida. The effects were dramatic, with the time trajectory of oxycodone deaths mirroring that of oxycodone supply.
On the one hand, this circumstantial evidence suggests that supply-side interventions against prescription opioids can have dramatic effects. On the other hand, Florida may have been experiencing a uniquely bad baseline situation in 2010 that may never again be replicated. Examining Texas's pill mill law, for example, Lyapustina and colleagues (2016) found reductions in the number of opioid prescriptions, number of pills dispensed, opioid volume, and average morphine-equivalent dose per transaction, but the reductions were 8–24 percent, not the enormous reductions seen in Florida. Overall, although further research is warranted, limited evidence suggests that state and local interventions aimed at reducing the supply of prescription opioids in the community may be effective. It should be emphasized, however, that none of these studies investigated the impact of reduced access on the well-being of individuals suffering from pain whose access to opioids was curtailed.
STRATEGIES FOR INFLUENCING PRESCRIBING PRACTICES
Reducing prescribing of opioids is at once a tool both for reducing lawful supply (by limiting the indications for prescribing them or otherwise reducing the number of patients holding prescriptions) and for reducing demand, or aggregate desire for using or misusing the drugs. Reduced prescribing can affect demand in two ways: first, by reducing patients' reliance on opioids to manage pain by satisfying their needs through other forms of pain management; and second, by reducing the number of patients or others who develop OUD and increasing the incentive for treatment among patients with OUD. This section describes a range of formal and informal policies, interventions, and tools designed to shape, guide, and regulate the prescribing practices of physicians and other health care professionals (the gatekeepers) authorized to prescribe these drugs.
Provider Education
The relief of pain represents one of the primary responsibilities of the practice of medicine (Federation of State Medical Boards, 2013). As detailed in this section, the breadth and depth of educational efforts to train physicians, nurses, pharmacists, occupational/physical therapists, and other health professionals have often fallen short of their goals for developing appropriate clinical competencies in pain management. Compared with the progressive advancement of medical education surrounding such fields as cardiology and oncology, advances in pain management education are entirely absent or minimally developed—often limited to a few hours of didactic lectures over multiple years of training.
Although detailed protocols have been developed through rigorous clinical trials for specific conditions (e.g., in the treatment of chest pain as a result of ischemic heart disease), the management of chronic noncancer pain has no equivalent foundation. Moreover, no single entity or organization has overall jurisdiction for the development of pain management guidelines, clinical pain competencies, or opioid prescribing practices. What exists appears to be a group of loosely aligned efforts sponsored by federal, state, and local agencies surrounded by professional organizations and private industry influences. These efforts are summarized below for their respective agencies and organizations.
U.S. Food and Drug Administration
Known by its modern name since 1930, the FDA is the oldest consumer protection agency in the U.S. federal government (FDA, 2015b). Building on its key milestone, the 1906 legislation that outlawed adulterated and misbranded food and drugs, the FDA has grown in scope and size to ensure the health and safety of a broad range of therapeutics, including opioid and nonopioid analgesics. As detailed in Chapter 6, the FDA reviews and approves new and reformulated drugs for use for defined medical indications. Importantly, it can also serve as a hub for advanced training (FDA, 2016a), including the opioid-specific Risk Evaluation and Mitigation Strategy (REMS), as part of an effort to reduce “risks of serious adverse outcomes including addiction, unintentional overdose, and death” (p. 2) from prescription opioid analgesics (FDA, 2017b). Notably, provider participation in the educational component of the opioid REMS is currently voluntary, with unclear evidence of reduction in opioid-related harms or impacts on opioid prescribing (FDA, 2016b). See Chapter 6 for further discussion of the role the FDA's REMS can serve in ensuring that the benefits of prescription opioids continue to outweigh their risks.
U.S. Centers for Disease Control and Prevention
The publication of the CDC Guideline for Prescribing Opioids for Chronic Pain (Dowell et al., 2016) may well represent a watershed moment in the education of health care providers in the management of chronic pain, and specifically with respect to the prescribing practices for opioid analgesics. As discussed later in this chapter, this guideline, in whole or in part, is being integrated into a wide range of educational resources (e.g., guidance from state-level medical boards). It is too early to understand its impact on changes in the quality of pain management or on opioid analgesic prescribing practices. Directed research could track such outcomes, especially as components of the guideline are incorporated into various educational materials at the undergraduate and postgraduate levels, as well as for the public at large. Concerns exist surrounding the proper interpretation of certain aspects of the guideline, especially with respect to the potential restriction of opioids for acute and/or chronic painful conditions. As discussed later in this chapter, patient-centered management, aided by patient educational materials explaining the risks and benefits of long-term opioid use, could be useful in optimal clinical use of the guideline.
National Institutes of Health (NIH)
As discussed in Chapter 3, NIH support for research and educational aspects of pain management is disproportionately small relative to, for example, HIV research. However, in the face of this disparity in resources to support the development of advanced pain care and address the opioid epidemic, small but determined efforts exist within NIH in support of pain research and education.
As a result of a 1996 congressional mandate, for example, the NIH Pain Consortium, including representatives from 24 NIH institutes and centers, was established to coordinate pain research and disseminate its findings. Subsequently, the consortium held a workshop in 2010 on the state of pain education in the United States to help establish a way forward for the future of education for health care providers (medical, dental, nursing, and pharmacy). The findings of this meeting were as alarming then as they are now: the consortium concluded that the nation is failing to properly educate and train the next generation(s) of health care providers entrusted with relieving pain. Then as now, medical students were receiving on average only 8 hours of training in how to measure, diagnose, and treat pain. A consequence of this failure in education is that pain often goes poorly treated, with some patients receiving the wrong treatment and/or medications. Some may receive too little, while others receive more than is warranted, for unspecified durations, and without the benefit of long-term follow-up to abate the risks of addiction or ensure that the plan is safe and effective. Sometimes, unfortunately, the result is OUD and its sequelae.
In response to this systematic failure, an NIH initiative, the Centers of Excellence in Pain Education (CoEPEs) (NIH, 2017), led by the National Institute on Drug Abuse (NIDA), was launched to increase pain education in medical, nursing, pharmacy, and dental schools across the nation. The plan for these centers was intended to support “pain education champions” and their teams in health care schools who have previously demonstrated a commitment to increasing pain education in their institutions. One of the key elements of this initiative is the production of interactive teaching tools, which other institutions can freely download and use to teach their students about pain and its treatment. An example of these modules can be found on the Pain Consortium website.12 While these efforts are ongoing and were initially met with great enthusiasm, budgetary restrictions and inconsistent funding sources have progressively undermined the initiative's strength and productivity. Strengthening and expanding this critical effort represents a key opportunity for NIH to support education surrounding opioid analgesia.
The challenge of supporting a national strategy for pain education is surprising in the face of the current opioid epidemic, as well as the recommendations of the Institute of Medicine (IOM) report Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (IOM, 2011). Resulting from a study conducted shortly after the passage of the Patient Protection and Affordable Care Act (ACA), that report offers specific recommendations to (1) improve curriculum and education in pain management for health care professionals, and (2) increase the number of health professionals with advanced expertise in pain care. Collaborative actions with other government agencies—for example, the Substance Abuse and Mental Health Services Administration (SAMHSA)—which has developed treatment improvement protocols such as Treatment Improvement Protocol 54 (TIP 54), Managing Chronic Pain in Adults with or in Recovery from Substance Use Disorders—could provide synergy for such educational efforts (SAMHSA, 2012).
Public and Private Universities/Professional Schools
Medical school education has been undergoing a transformation nationwide, requiring a complete redesign of curriculum to incorporate the early integration of clinical encounters, development of an interdisciplinary team approach to care models, and development of clinical competencies prior to graduation (Satterfield et al., 2004). Despite this redesign, however, the tradition of pain management education in undergraduate curriculum has often been more robust in other disciplines, such as pharmacy, dentistry, nursing, and veterinary schools, relative to medicine. In fact, according to one study, topics related to pain pathophysiology and management appear to be more developed in the training of physician assistants than in that of physicians (Doorenbos et al., 2013).
In the past, the limited hours dedicated to pain management education in medical schools have been restricted to a series of didactic lectures given in the first year. This approach has been evolving in recent years so that students are increasingly challenged with clinically relevant reenactments. An example is the “Danovic” case at the University of California, San Francisco, which is presented early in the first-year curriculum (UCSF, 2017). In this case, Mr. Danovic has a history of chronic low back pain that provides multiple opportunities to develop longitudinal interdisciplinary links for his pain management throughout the subsequent 4 years of training and to integrate aspects of other pain management learning. Additional curriculum advances include the Bridges program, based on “inquiry” (i.e., posing questions or scenarios to students as opposed to presenting facts), which emphasizes a systems approach to care. Numerous similar innovations, such as the learning models developed by the Academy of Medical Educators (AoME, 2017), are occurring across the country. These integrated programs represent a broad opportunity for the expansion of pain curriculum at the nation's medical schools. They may also partially offset the influence of industry representatives that often inadvertently fill gaps in undergraduate medical education around prescribing practices (Relman, 2001).
Taken together, undergraduate medical education that integrates longitudinal, inquiry-based curriculum and that stresses interactive sessions over large lecture formats has the potential to greatly improve clinical care delivery for pain through improved communication and clinical competencies. Additionally, the development of integrated topic pathways may improve the teaching of and competency in pain management by replacing traditional topic silos during the third-year core clerkships (Poncelet et al., 2011). Such approaches are intended to break down traditional communication barriers and empower health care providers to embrace an interprofessional model of care that includes pain management—a model that increases the likelihood that all members of a treatment team will advise clinicians to use both pharmacologic and nonpharmacologic alternatives, including multimodal adjuvant therapies (e.g., physical therapy, acupuncture, manipulation or massage, ice, and music therapy). In addition to efforts sponsored by individual professional schools, it may be hoped that modules developed through the NIH CoEPEs (discussed above) will allow additional pain education resources to be made available and introduced throughout any professional health care program.
Professional Societies
Despite the prominence and availability of Web-based patient care guidelines for the management of pain, whether issued by national or international professional societies (e.g., American Academy of Pain Medicine, American Pain Society, International Association for the Study of Pain), the under- and overtreatment of pain remains a widespread challenge. Although such societies may provide a wealth of information through online modules, annual meetings, and seminars, they are often targeting health care providers who are already engaged in pain management and/or the treatment of OUD. Primary care physicians, often represented by such organizations as the American Academy of Family Physicians, care for the vast majority of patients with acute and chronic pain, but may not be directly connected to or engaged in these pain society resources and thus must develop and provide their own educational resources for pain management (see, for example, AAFP, 2017).
Depending on their participation in such educational initiatives, the majority of physicians likely have practice and knowledge gaps that include inadequate understanding of pain assessment and diagnosis, especially in the context of chronic pain; inappropriate use of analgesic medications; failure to assess and reassess pain systematically and in the context of opioid use; and the inability to distinguish among opioid tolerance, physical dependence, and OUD (Murnion et al., 2010). Just as interprofessional approaches to undergraduate education have emerged, pain and addiction societies could work more closely with organizations supporting primary care providers, as well as seek to find the correct balance of industry sponsorship that does not unduly bias their educational content (Relman, 2001).
State Medical Boards (SMBs)
SMBs are the primary regulatory authority governing physician prescribers of opioids, through the provision/renewal of medical licensure and related functions (e.g., disciplinary actions related to inappropriate prescribing). To varying degrees, SMBs also serve as an educational resource for clinicians in their state through the publication of relevant legal information (e.g., the statutory obligations for prescribers of controlled substances) or the dissemination of best practice guidelines (discussed later in this chapter). In the context of pain management and opioid prescribing practices, this constellation of state-level oversight represents both a powerful tool to assist physicians in providing safe and effective care and a potential source of variability in the broader guidance to physicians across the country.
Summary
Current efforts to improve prescriber pain education and knowledge about prescription opioid misuse, such as the NIH CoEPEs, are inadequate and at risk of collapsing. Providers managing pain are often left to pick and choose from weakly supported alternatives. Addressing this lack of alternatives is a topic discussed in Chapter 3. However, any meaningful effort to improve pain management will require a fundamental paradigm shift in the nation's approach to mandating pain-related medical education; completion of a brief online module will not be sufficient (Holliday et al., 2017). The committee recommends that state medical schools and other health professional schools coordinate with their state licensing boards for health professionals (e.g., physicians, nurses, dentists, pharmacists), the National Institutes of Health's Pain Consortium, the U.S. Food and Drug Administration, the U.S. Centers for Disease Control and Prevention, and the U.S. Drug Enforcement Administration to develop an evidence-based national approach to pain education encompassing pharmacologic and nonpharmacologic treatments and educational materials on opioid prescribing (Recommendation 5-2).
Prescribing Guidelines
As summarized in a Chapter 2, there are many medical situations in which opioids might be considered an appropriate treatment option. The most common indications include (1) acute pain management, such as after injury; (2) management of pain in the context of cancer or the end of life when accompanied by pain; and (3) management of chronic pain not due to a malignancy. Federal, state, and professional organizations have issued clinical guidelines for the use of opioids (e.g., initiation, dosing, monitoring, discontinuation) in each of these situations. The issuance of these guidelines often is accompanied by such efforts as educational outreach, including continuing medical education (CME), to foster implementation (Haegerich, 2016).
Opioids and Acute Pain Management
Acute pain is experienced commonly after surgical or dental procedures, traumatic injuries, and some normally transient medical conditions (e.g., acute low back pain) when its resolution is expected over a time course of hours to several weeks. Depending on the specific situation, opioids, nonopioid medications, nerve blocks, topical medications, and other measures might be used individually or combined in a multimodal approach (see Chapter 2). As discussed in previous chapters, understanding and controlling opioid use in these situations is important as these routes of exposure may lead to long-term use, particularly in certain populations (Sun et al., 2016; Webster et al., 2007). Additionally, as detailed earlier, unused medications provided by hospitals, emergency rooms, and clinics may leak into the community and be used for nonmedical purposes (Inciardi et al., 2007).
The subject of guidelines for acute pain management currently revolves primarily around use rather than dosage or duration. Dosage guidelines are widely available and fairly widely accepted. However, opioids prescribed for acute pain syndromes have too often been provided at doses and dosing intervals and for durations unlikely to yield optimal effects (Humphries et al., 1997). One attempt at providing general guidelines for the use of opioids for acute pain was made by the Utah Department of Health,13 and portions of these guidelines have been incorporated into the guidelines used by other states. The process of developing the guidelines involved broad representation of stakeholders on advisory and working groups. These guidelines call for opioids to be used only when nonopioid alternatives are deemed inappropriate, and for the drugs to be issued in carefully limited amounts (in dosage and duration) and after education of the patient concerning appropriate use and storage.
Various groups have independently developed guidelines for the prescribing of opioids for management of acute pain in emergency rooms (del Portal et al., 2016) and for the management of pain in acutely injured workers (Mai et al., 2015). In one study, del Portal and colleagues (2016) found that opioid prescribing decreased significantly in an acute care setting (from 52.7 percent before the guideline was issued to 29.8 percent immediately after its introduction, and to 33.8 percent 12 to 18 months later) based on retrospective chart review for more than 13,000 patient visits. There do not appear to be any widely accepted guidelines for postoperative opioid prescribing, although one study found that the amount of opioid provided often was much larger than the amount required (Hill et al., 2017). The suggestion recently was made that postoperative opioid prescribing be based on the specific surgical procedure, type of anesthesia used, patient age, and other variables (Kim et al., 2016).
Guidelines for the management of back pain issued in 2017 by the American College of Physicians suggest using nonpharmacologic approaches for treatment of acute and subacute back pain, given that this type of pain often resolves on its own over time. When pharmacologic treatment for acute and subacute back pain is desired, the guidelines suggest the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or skeletal muscle relaxants (Qaseem et al., 2017).
Opioids and Pain Management in the Context of Cancer and End of Life
The use of opioids for the treatment of pain in the context of cancer and end of life is broadly supported by outcome studies. While not adequately effective as sole analgesic agents in every patient, opioids, including morphine, oxycodone, fentanyl, and others, can reduce pain due to malignancies, including so-called breakthrough pain, a sometimes severe form of cancer pain of very rapid onset (Zeppetella and Davies, 2013). The use of opioids for cancer pain is codified in the World Health Organization's (WHO's) analgesic ladder, one of the oldest and most widely accepted sets of opioid treatment guidelines (WHO, 1986). Regrettably, 10–20 percent of cancer patients experience pain that is refractory to standard opioid management. For these patients, a number of opioid- and nonopioid-based options have been described, but evidence is not yet sufficient to develop guidelines for their use (Afsharimani et al., 2015).
A number of studies have estimated compliance with cancer pain management guidelines. The results suggest that, despite the existence of various guidelines, pain assessment and reassessment and some other provisions of the guidelines are not always adhered to, and that pain control can be improved when guidelines are followed (Du Pen et al., 1999; Mearis et al., 2014; Miaskowski et al., 2001). On the other hand, many more people are surviving cancer treatment than was the case during the development of the WHO guidelines. It is unclear what role opioids should play in the management of persistent pain after successful cancer treatment that might be due to surgery, chemotherapy, radiation, or other related causes.
Opioids and Pain Management in the Context of Chronic Pain
The controversial nature of the practice of using opioids to treat chronic pain, as well as growing recognition of its adverse consequences for both individual patients and society, has prompted the development of numerous prescribing guidelines. These guidelines have been sponsored and promulgated by professional societies; SMBs (such as the Federation of State Medical Boards); and federal agencies, such as the CDC.
Of the sets of opioid prescribing guidelines currently available, that developed by the CDC is the most recent, comprehensive, and influential (Dowell et al., 2016). The CDC's inclusive process for developing the guideline emphasized the use of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology to rate the quality of the evidence used in constructing the guideline, as well as the strength of the resulting recommendations. This process further involved the engagement of federal partners that included representatives from SAMHSA, NIDA, the FDA, the U.S. Department of Veterans Affairs (VA), the U.S. Department of Defense (DoD), and others. The development process further involved constituents, including clinicians and patients. Peer review of the guideline was solicited, as were public comments. The 12 key provisions of the resulting guideline (see Box 5-3) emphasize consideration of nonopioid options prior to or in addition to opioids, careful pre-prescribing risk stratification, conservative dosing, careful follow-up, and appropriate discontinuation/tapering.
Because the CDC guideline was issued only recently, its impact on prescribing practices remains unknown. Some have questioned the strength of the data behind some of the recommendations, such as the overall emphasis on improvement in function, as well as in pain control, in the consideration of whether benefits of using the drugs are expected to outweigh risks to the patient (Pergolizzi et al., 2016).
BOX 5-3
The U.S. Centers for Disease Control and Prevention's Recommendations for Prescribing Opioids for Chronic Pain Outside of Active Cancer, Palliative, and End-of-Life Care.
With respect to other guidelines for chronic pain management that have been in the field longer than the CDC guideline, researchers have found modest improvement in practice behaviors, such as use of urine drug screens and referral for specialty evaluation, and modest impacts on overall opioid prescribing rates, as well as overdose rates (e.g., Barber et al., 2017; Beaudoin et al., 2016; Chen et al., 2016). Moreover, strong state-level guidelines were associated with a reduction in the number of patients receiving high doses of opioids (Garg et al., 2013; Sullivan et al., 2016). Notably, multipronged efforts that include guidelines as well as other educational information for providers on how to prescribe opioids safely have been found to be associated with decreases in emergency department visits and deaths from opioid overdose (Cochella and Bateman, 2011; Paone et al., 2015). These findings suggest that guidelines may be able to moderate the most aggressive opioid prescribing but are unlikely to be sufficient on their own to ensure the application of optimal medical practices in all cases, and that multipronged educational interventions and changes in reimbursement for pain management are required.
State Medical Board Guidelines
In an attempt to provide educational resources on the topic of pain management and opioid prescribing practices, many SMBs have either developed their own best practices preceding the release of the CDC guideline in 2016 or subsequently responded by incorporating foundational components of that guideline addressing key decisions encountered during clinical pain management. Although the CDC guideline was intended to serve as a broad resource for primary care physicians, it is being adapted and largely interpreted at the state level for all practicing physicians across the nation. A brief review of three key CDC topic areas across the Web-based resources of five SMBs (California, Florida, Kentucky, Ohio, and Washington) on pain management and opioid prescribing practice reveals examples of content variability:
- Determining when to initiate or continue opioid treatment—California's guidance on initiation of opioid therapy for chronic pain references carefully defined, 90-day opioid trials (MBC, 2014), whereas Ohio's SMB cautions against using opioids to treat chronic pain but advises clinician vigilance should they be deemed necessary (GCOAT, 2013).
- Opioid selection, dosing, and duration—Advice generally echoes the CDC's “start low and go slow” approach; however, different morphine-equivalent doses are specifically cited by different SMB documents. California mentions 80 mg/day as a threshold above which caution should be used (MBC, 2014), while a joint publication from the Washington State Agency Medical Directors' Group urges caution at any dose, and additionally recommends referral to specialists for cases necessitating doses above 120 mg/day (WSAMDG, 2015).
- Follow-up, monitoring, and discontinuation of opioid treatment—Other areas of variation include whether and how to use treatment agreements and screening tools for OUD risk (discussed in Chapter 2), as well as considerations for monitoring patients on long-term opioid therapy and conditions necessitating treatment discontinuation. Perhaps most important is the degree to which guidance regarding tapering of opioid treatment is provided. SMBs vary in the depth to which this issue is addressed, from simply recommending referral to addiction or pain specialists (Ohio), to describing the risks, benefits, and management of withdrawal symptoms associated with various weekly reductions in opioid use (California and Washington).
Most of the selected SMBs that provide opioid guidance documents recommend consideration of nonopioid/nonpharmacologic pain management strategies prior to initiation of opioid therapy, and contain appendixes varying in number and length providing supplemental data for prescribers and patients. Many of the documents also recommend that opioids for acute pain be prescribed in limited amounts and doses consistent with the expected clinical course of the case (such as postsurgical pain). Such state-to-state variation is to be expected, and often is due to the goal of the particular guidance document (e.g., Washington's guidance focuses on pain management broadly, whereas Ohio has separate documents for chronic and acute pain, with comparatively little emphasis on patient education or other “wraparound” services). States also may vary in the degree of autonomy that is customary among their physicians.
Unfortunately, in some cases, SMB guidance for opioid pain management can be quite limited, describing only the statutory obligations of physicians prescribing controlled substances for pain, although reference also may be made to the CDC guideline (FBM, 2010; KBML, 2003). In short, there are wide disparities in the availability and comprehensiveness of SMBs' prescribing guidance. In April 2017, the Federation of State Medical Boards (FSMB) released a revised “model policy” for chronic use of opioid analgesics (FSMB, 2017), for use by SMBs seeking to evaluate physician management of patients with pain. This guidance is largely consistent with (if not as broad and comprehensive as) the CDC guideline. Notably, FSMB stresses at the outset that “effective means of achieving the goals of these Guidelines vary widely depending on the type and causes of the patient's pain, the preferences of the clinician and the patient, the resources available at the time of care, and other concurrent issues beyond the scope of these Guidelines” (FSMB, 2017, p. 2).
In summary, prescribing guidelines may be able to improve provider prescribing behavior but may be most effective when accompanied by provider education and other measures designed to facilitate implementation.
Electronic Medical Records and Decision Support
The use of electronic medical record (EMR) systems is expanding rapidly in both inpatient and outpatient medical settings. Use of EMRs was led by the VA, but aggressive federal policies have prodded many offices, clinics, hospitals, and integrated health care systems to employ the technology. Clinic notes, study results, laboratory values, pharmacy information, and other key data may be included. Compared with more traditional paper-based systems, EMRs offer potential improvements to health care delivery, including but not limited to increased efficiency, better adherence to guidelines and regimens, and fewer medical errors and related events (Campanella et al., 2016).
These advantages could contribute to safer and more effective opioid prescribing for several reasons. First, notes documenting treatment and follow-up plans may be more easily located by consulting an EMR than by sorting through paper files, and delays in accessing the records are minimized when providers need patient information quickly. Importantly, EMR systems containing sections for current medications, allergies, and other pharmacy-related information (e.g., last medication refill dates and tablet quantities) may aid greatly in managing higher-risk patients. The electronic format is conducive to the use of treatment templates in which opioid follow-up assessments and ongoing prescribing plans can be included.
At present, a modest amount of information helps inform the utility of EMRs and opioid prescribing in different settings. A pre/postimplementation analysis concluded that the implementation of an EMR system may have contributed to higher rates of signed opioid treatment agreements, use of urine drug screens, and documentation of assessment of functional status (Anderson et al., 2016). Another study demonstrated that the inclusion of electronic alerts for the presence of opioid-use care plans within an EMR system may reduce opioid prescribing by emergency departments for high-frequency emergency department patients (Rathlev et al., 2016). Use of EMRs, however, may not always discourage opioid prescribing. A regression analysis to analyze the prescribing behavior of primary care physicians with and without EMR systems showed that visits to physicians with EMRs were more likely to result in opioids being prescribed relative to visits to physicians using more traditional systems (Harle et al., 2014).
Evidence on the effectiveness of clinical decision support systems (CDSSs) for opioids, incorporated within EMRs, is similarly conflicting. Trafton and colleagues (2010) describe a commendable attempt to iteratively improve and deploy a CDSS for primary care physicians treating chronic pain with opioids. In the end, while the CDSS did overcome some perceived barriers to guideline adherence (e.g., medication selection, dosing calculations), remaining systemic barriers at the health care system level (e.g., lack of time, competing clinical demands) appear to have blunted the beneficial impact of the CDSS on patient outcomes (Trafton et al., 2010). Thus, the impact of electronic and other types of record-keeping systems on pain management or opioid prescribing, whether positive or negative, is not yet fully understood.
Insurer Policies for Pain Management
Insurer policies have a large and logical impact on health care delivery through their considerable financial leverage with respect to covering and reimbursing for specific clinical services or restricting access to others. In pain management, for example, a policy may or may not require specified indications before reimbursement for prescription opioids is authorized; in contrast, other policies may have more stringent requirements for authorization of nonopioid pain therapies and/or inadequate reimbursement structures. These policies, in turn, may result in marked differences in access to services and in desired outcomes. Insurers, including sources of publicly funded health care coverage and pharmacy benefit managers, therefore can play a critical role in shaping clinical practices related to opioids and nonopioid alternatives for pain management. As a result of increasing recognition of the role such policies can play in improving analgesic care, examples are emerging of both reductions in inappropriate opioid prescribing and enhanced access to more comprehensive models of pain management.
Opioid Prescribing Policies
Haegerich and colleagues (2014) reviewed eight studies examining the effect of patient review and restriction (PRR) (i.e., “lock-in”) programs on opioid use. PRRs, used by public and private insurance plans, may require patients suspected of misusing controlled substances to obtain prescriptions from a specified prescriber and/or pharmacy. Overall, the findings of this review are impressive. Four of the studies considered both cost savings and health outcomes. These studies generally found that in the four respective programs studied (in Louisiana, Ohio, Oklahoma, and Washington), PRRs were associated with reductions in opioid use of one-third to one-half and with reductions in the number of patients able to successfully access multiple providers or pharmacies. The Washington study, which followed up patients 1 year later, also found significant reductions in emergency department and physician visits and in hospital costs (Haegerich et al., 2014). PDMP data can be used to determine whether a PRR is needed. In a survey of state Medicaid agencies, however, 48 percent (22 states) reported that their fee-for-service PRR program does not have access to the state PDMP (Pew Charitable Trusts, 2016).
Four studies reviewed by Haegerich and colleagues (2014) examined drug utilization review (DUR) programs that review claims data to identify and notify providers of potentially problematic use patterns. Although none of these four studies evaluated health outcomes, all found reductions in drug utilization, and one RCT found reductions in numbers of prescribers and pharmacies used. In a later study, Qureshi and colleagues (2015), utilizing pharmacy claims data from 980 members enrolled in a commercial health plan who met DUR criteria, found a 28.1 percent reduction in potentially unsafe combination therapy involving opioids and other central nervous system drugs (benzodiazepines or antidepressants). State Medicaid programs have implemented the use of DUR to curb inappropriate opioid prescribing.
Finally, Haegerich and colleagues (2014) also examined studies on prior authorization (PA) and quantity limit (QL) programs. PA requires review of medical justifications before drugs are covered by an insurer, while QL limits the amount of a drug that can be dispensed in a given time frame. Haegerich and colleagues (2014) summarize the finding of Morden and colleagues (2008) that the 21 states that implemented PA in their Medicaid programs saw 34 percent reductions in oxycodone use over the study period, whereas those with more lenient PA policies witnessed a slight (but nonsignificant) increase. Three studies of PA and QL by Oregon State University are described as finding significant reductions in use of long-acting (LA) opioids and carisoprodol, but no significant impact on sedatives/hypnotics (Haegerich et al., 2014).
In summary, insurance-based policies, such as those involving PRR, DUR, PA, and QL, have substantial potential to reduce the use of specific prescription drugs, although their impact on health outcomes remains uncertain.
Coverage and Reimbursement of Nonopioid Pain Management
As discussed in Chapter 2, there are multiple nonopioid pharmacologic (e.g., NSAIDs) and nonpharmacologic (e.g., physical therapy, cognitive-behavioral therapy) options available for patients with chronic pain. Nevertheless, insurer policies affect access to and uptake of these treatment options. The IOM report Relieving Pain in America specifically points to misaligned incentives in fee-for-service insurance systems as a primary obstacle to comprehensive and effective pain management, citing lower (or absent) reimbursement of psychosocial or nonprocedural treatments (IOM, 2011).
In part in response to the growing opioid epidemic, some insurers and state Medicaid agencies are working to expand access to nonopioid pain management services for common clinical indications, such as back pain (Cigna, 2016; McLaughlin, 2015; Oregon Health Plan, 2016). This is occurring despite the relatively lower cost of opioid prescriptions, which carry an average out-of-pocket cost of $10 per prescription (although the cost of ER formulations can be more than double that of immediate-release [IR] formulations) (Craig and Strassels, 2010). While relatively more expensive in the short term, integrated or multidisciplinary pain treatment programs have demonstrated long-term cost-effectiveness and increased functional improvement for patients (Turk and Burwinkle, 2005). Promising clinical research into opioid dose reduction programs, more comprehensive pain management, and the effectiveness of nonopioid treatments for pain is discussed further in Chapter 3.
The judicious deployment of insurer policies related to opioid prescribing, outlined above, would logically benefit from a commensurate increase in coverage of and access to nonopioid pain management. This broader approach to pain management is consistent with the guidelines of the CDC (discussed earlier in this chapter), the American College of Physicians, and FSMB, among others, that recommend careful initiation of opioids in the context of a comprehensive pain management plan (Dowell et al., 2016; FSMB, 2017; Qaseem et al., 2017). Accordingly, the committee recommends that public and private payers develop reimbursement models that support evidence-based and cost-effective comprehensive pain management encompassing both pharmacologic and nonpharmacologic treatment modalities (Recommendation 5-3).
Prescription Drug Monitoring Programs
PDMPs, currently authorized in every U.S. state except Missouri,14 as well as in the District of Columbia and the U.S. territory of Guam (Brandeis PDMP TTAC, 2017), are statewide electronic databases designed to prevent diversion and misuse of controlled substances. They require pharmacies and sometimes dispensing physicians to submit to a central office data on controlled substances prescribed and dispensed (e.g., drug type, dose, amount dispensed) (Haegerich, 2016), as well as insurance/payment and patient information. These data can be monitored for patterns in prescribing and dispensing. This monitoring for patterns includes the identification of possible “doctor shoppers” (individuals who visit multiple prescribers or pharmacies to obtain multiple prescriptions), as well as need for treatment, unsafe drug combinations, and inappropriate provider prescribing practices (Brandeis PMP COE, 2012, 2013, 2014; Jann et al., 2014; Patrick et al., 2016). Because PDMPs include virtually all data on prescriptions dispensed to a patient regardless of payment method, they allow for more complete monitoring than claims databases, which often are limited to data on payments for prescriptions within a particular network (Brandeis PMP COE, 2013).
States vary somewhat in terms of authorized users and recipients of PDMP data (NAMSDL, 2016). In most states, PDMPs are administered by health departments, boards of pharmacy, or a single state authority. Other states' programs are administered by law enforcement agencies, boards of pharmacy in conjunction with other agencies, professional licensing boards, or departments of consumer protection/affairs. As of May 2016, however, in only a handful of states (New Mexico, New York, Ohio, Oklahoma, Utah, and Vermont) were departments of health or commissioners of public safety authorized users of PDMPs, meaning that they are permitted to request and receive information on behalf of agency activities (Davis et al., 2015; Haegerich, 2016; NAMSDL, 2016). Prescribers and dispensers and physician assistants/medical residents/nurse practitioners are authorized recipients of PDMP data in every state, and law enforcement officials are authorized recipients in all but one state (Nebraska). Table 5-1 shows other types of professionals who are authorized users by state. As is shown, several states do not permit access for mental health and substance use and other types of professionals who could potentially use the data to monitor opioid use and related harms.
Although they have operating PDMPs, some states have laws that do not expressly mandate that prescribers and/or dispensers access PDMP information.15 Most states are permitted to share PDMP data with other state PDMPs and/or with authorized users in other states (NAMSDL, 2016).
With respect to effects on prescribing practice and patient receipt of drugs from multiple health care providers, PDMPs are currently considered promising strategies based on before–after studies and time series analysis (Haegerich, 2016). A contextual review conducted to support development of the CDC's Guideline for Prescribing Opioids for Chronic Pain concluded that there is indirect evidence for the utility of PDMP data for identifying indicators of risky opioid-taking behaviors and prescribing practices (Dowell et al., 2016). A recent analysis of Medicaid data suggests that mandatory prescriber registration with state PDMPs (as opposed to mandatory use of them) can lead to decreased prescribing of Schedule II opioids, although whether this resulted in safer prescribing or limited access to legitimate pain relief could not be assessed (Wen et al., 2017). In patients for whom a decision is made to initiate or continue opioid therapy, the CDC guideline recommends that clinicians review PDMP data for high-risk drug combinations or dosages (see Box 5-3, presented earlier). Furthermore, the guideline states that PDMP data should be reviewed “when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months” (Dowell et al., 2016, p. 1639).
TABLE 5-1
States Authorizing Use of PDMP Data, by Selected Professions (as of May 2016).
Research on the effectiveness of specific features of PDMPs is currently limited (Patrick et al., 2016). The Brandeis Prescription Monitoring Program Center of Excellence identified PDMP best practices based on a systematic review of articles published through November 2011 (Clark et al., 2013). None of the studies met criteria for the highest level of evidence (RCT or meta-analysis). Best practices based on the next level of evidence (observational study with comparison group) included using serialized prescription forms and sending unsolicited reports and alerts to prescribers, pharmacists, investigative agencies, and other relevant parties regarding questionable activity (Clark et al., 2013). Current laws in most states allow for unsolicited reporting but vary somewhat in terms of the parties to whom the reports may be provided (NAMSDL, 2016) (see Figure 5-2). Generally, data support the effectiveness of PDMPs in reducing the supply of prescribed controlled substances in the community, which is one, but not the only, causal factor in the risk of OUD and overdose.
Some states have worked to share PDMP data with other programs to support monitoring of prescribing patterns. Washington State's PDMP, for example, shares data with state Medicaid and workers' compensation programs to provide a more complete picture of controlled substances prescribed to patients. State program administrators reported that this effort supported improved identification of and early intervention for patients at risk for substance use disorder and overdose, led to reductions in costs associated with unnecessary prescription drug use and diversion and uncoordinated care, and improved education of prescribers about PDMPs, among other positive effects (Brandeis PMP COE, 2013). These findings are not specific to opioids, however. In Ohio and other states, a risk score (the NARxCHECK) that provides an assessment of patients' history of use of controlled substances based on PDMP data is incorporated into EMRs to support intervention efforts.

FIGURE 5-2
Unsolicited reporting of prescription drug monitoring program (PDMP) data to prescribers, dispensers, licensing boards, and law enforcement (as of May 2016). SOURCE: NAMSDL, 2016.
As noted above, states also utilize PDMP data to address at-risk prescribing through use of such tools as prescriber report cards and reports to licensing boards and law enforcement. Data on how these reports impact prescribing practices are currently limited, however. In Arizona, report cards summarizing prescribing over the past year were sent to outlier prescribers (those for whom PDMP data indicated that the number or total dosage units prescribed were 1 standard deviation above the average in their specialty and county). Preliminary findings for a county 1 year following implementation of the report cards show that the percentage of outlier prescribers fell from 19.2 to 14.2 percent (Brandeis PMP COE, 2014). In such states as Kentucky and Texas, provision to investigators of information regarding problem prescribers is believed to have helped identify and address this problem, both through removal and by providers being encouraged to modify their prescribing practices (Brandeis PMP COE, 2014).
As noted earlier, some states allow substance use and mental health professionals to access PDMP data. In treatment settings, the data may be used to check whether patients are being prescribed controlled substances. Limited evidence suggests that such access by these professionals may play a role in reducing opioid use by individuals in treatment (Brandeis PMP COE, 2015). It is worth noting that federal law itself may pose an additional obstacle related to treatment for substance use disorder: 42 C.F.R. Part 2 prohibits PDMP data from including any information related to substance use disorder services (e.g., receipt of methadone from an opioid treatment program). This provision carves out an additional area of patient privacy, often a contentious issue surrounding PDMPs, but necessarily excludes potentially relevant information from the PDMP.
By reducing the availability of opioids from medical sources in the community, one might reasonably expect that PDMPs would reduce mortality from opioid overdose. Yet relatively few studies have evaluated the impact of PDMPs on opioid-related mortality, and the results of available studies are mixed (Delcher et al., 2015). An analysis of observational data for the period 1999 to 2005 found no significant differences in rates of opioid overdose mortality and rates of opioid drug use between states with and without PDMPs (Paulozzi et al., 2011). However, PDMPs vary so widely in their legal requirements that little effect would be expected in a “yes or no” comparison. Until recently, for example, PDMPs were used primarily for law enforcement rather than public health purposes in most states, so an effect on drug overdose mortality might not be expected unless their use for this purpose had been articulated (Green et al., 2011). Additionally, utilization of PDMPs by health care providers was not included when the impact of PDMPs on overdose mortality or opioid use was assessed in two studies (Green et al., 2011; Kerlikowske et al., 2011). In another study that evaluated mortality data in states and the District of Columbia with and without PDMPs during 1999–2008, implementation of PDMPs was found not to be associated with reductions in drug overdose mortality in most states (Li et al., 2014).
A time series, quasi-experimental study of Florida's PDMP found that oxycodone-caused mortality declined by 25 percent in the month after implementation of the PDMP in 2011. This finding was significant after controlling for declines in mortality associated with the introduction, before implementation of the PDMP, of tamper-resistant oxycodone hydrochloride (HCL) controlled-release tablets to the market; law enforcement efforts to crack down on pill mills; and stricter rules and regulations related to prescribing of controlled substances (Delcher et al., 2015). However, even the study authors acknowledge the complex interrelationship among variables in the study, and specifically mention their lack of an explanation for the PDMP's mechanism of influencing their reported outcome, calling it an “important [remaining] empirical question” (Delcher et al., 2015, p. 65). This may be because Florida circa 2010, as discussed earlier in this chapter, may have been a unique case study that does not generalize well to other states. Another recent analysis that included all state PDMPs found that implementation of a PDMP was associated with a reduction in opioid-related overdose deaths of 1.12 per 100,000 people in the year after implementation. Greater reductions in opioid-related overdose were observed in states where PDMPs included robust features, such as monitoring of greater numbers of drugs with abuse potential and at least weekly updating of PDMP data (Patrick et al., 2016). As of April 2017, the interval for PDMP data collection was within a week or less in all states except Alaska, which will go to weekly reporting starting in July 2017, and Montana (which reports data every 8 days). Only one state—Oklahoma—had real-time PDMP reporting as of April 2017 (NAMSDL, 2017).
Some researchers have noted that while PDMPs may have an important role to play in preventing opioid overdoses, a multipronged approach that includes PDMPs is needed to foster significant reductions by addressing multiple correlates (Davis et al., 2014). Explicit and public articulation of the application and role of PDMPs in overdose prevention may increase their effectiveness and use for this purpose (Green et al., 2015a).
In summary, evidence suggests that PDMPs can help address the opioid epidemic by allowing prescribers, dispensers, and other stakeholders to track prescribing and dispensing information. State laws differ widely in who has access to PDMP data, with some states denying access to certain stakeholders (e.g., substance use and mental health professionals, health departments) that could use the data to monitor opioid use and related harms. As noted earlier, some states do not require prescribers and/or dispensers to check PDMP information, assuming that a mandate would be overly burdensome and that the PDMP's availability is sufficient to enable responsible prescribing. As a result, PDMP data currently are not being used to their full potential.
The committee recommends that the U.S. Department of Health and Human Services, in concert with state organizations that administer prescription drug monitoring programs, conduct or sponsor research on how data from these programs can best be leveraged for patient safety (e.g., data on drug–drug interactions), for surveillance of policy and other interventions focused on controlled substances (e.g., data on trends in opioid prescribing, effects of prescriber guidelines), for health service planning (e.g., data on discrepancies in dispensing of medications for treatment of opioid use disorder), and for use in clinical care (i.e., in clinical decision making and patient–provider communication) (Recommendation 5-4).
STRATEGIES FOR REDUCING DEMAND
This section reviews strategies aimed at reducing aggregate desire and need for opioids, including both reducing patients' reliance on opioids for pain management and reducing the occurrence and prevalence of untreated OUD. Accordingly, the discussion encompasses two main strategies: education programs focusing on alternatives to opioids for pain management and prudent and limited use of opioids if they are prescribed; and health policies bolstering and improving access to and utilization of evidence-based treatment for OUD.
Patient Education
This section addresses targeted patient education programs as well as mass media campaigns for the general public.
Targeted Patient Education Programs
Patients' understanding of the potential benefits and risks of and alternatives to opioids can be influenced by targeted patient education programs, provider initiatives mediated by professional education, and disclosures by manufacturers mandated by the FDA. Unfortunately, research on the effectiveness of patient education in reducing the risk of harms from prescription opioids is lacking. In the review of evidence conducted to support development of the CDC Guideline for Prescribing Opioids for Chronic Pain, investigators found no studies evaluating the effectiveness of patient education as a risk mitigation strategy. However, evidence suggests that many patients lack knowledge about opioids, indicating a need for patient education (Dowell et al., 2016). The CDC guideline recommends that before initiating opioid therapy, clinicians and patients weigh the known risks and benefits, available alternatives, and mutual responsibilities for optimal therapy. In connection with its prescribing guideline, the CDC has prepared a number of informational materials for patients on opioids and the risks associated with their use, as well as pharmacologic and nonpharmacologic alternatives for pain management (CDC, 2016b).
Other organizations also have developed informational materials for patients to promote safe opioid use and awareness of alternative therapies, although studies have not been conducted to assess the effectiveness of these materials. In 2016, the FDA issued guidance for patients on what to ask their providers before taking opioids (FDA, 2016c). The guidance recommends that patients ask their providers why they might need the medications (including asking whether there are alternative medications they can take to help with pain relief), how long they should take them, and whether they should have a prescription for naloxone (FDA, 2016c).
The potential value of patient education for reducing opioid-related harms also is supported by a number of health care organizations. The VA/DoD Clinical Practice Guideline for Opioid Therapy for Chronic Pain recommends education about opioids and risk mitigation strategies for both patients and family members (VA and DoD, 2017). Pharmacists are trained to educate patients and others on the disposal of prescription medications, and the American Society of Health-System Pharmacists encourages pharmacists to educate patients about the storage, handling, and disposal of prescription medications (ASHP, 2011). A number of states' opioid prescribing guidelines also recommend education for patients on the risks and benefits of opioid therapy, alternative treatment options, and safe storage and disposal.
Part of the committee's charge was to describe education for patients (as well as prescribers) about safe storage and disposal of opioid medications as a means of curbing opioid-related harms. As discussed earlier in this chapter, many patients do not safely store and dispose of their prescription opioid medications, which can lead to misuse (Binswanger and Glanz, 2015; Reddy et al., 2014). Available studies that include a specific focus on the role of education in promoting safe storage and disposal of opioids are preliminary and have small sample sizes.
A pilot study of a brief, Web-based educational intervention found significant improvements in knowledge about safe storage and disposal of prescription opioids postintervention and at 1-month follow-up. The study also found reductions in self-reported misuse (e.g., saving pills, lending medications to others) 1 month postintervention (McCauley et al., 2013). The intervention, which presented safety information in an interactive multimedia format, was administered to 62 adult outpatients who presented for treatment of chronic pain at pain management and dental clinics (McCauley et al., 2013). Likewise, in a prospective study of 300 adult cancer outpatients, those provided with educational material on safe opioid use, storage, and disposal each time they received an opioid prescription were significantly less likely to have unused medication at home (38 versus 47 percent) and significantly more likely to keep their medications in a safe place (hidden, 75 versus 70 percent; locked, 14 versus 10 percent) relative to patients who did not receive such material. The study found further that patients receiving the intervention were significantly more aware of proper opioid disposal methods (76 versus 28 percent) and less likely to share their opioids with others (3 versus 8 percent) (de la Cruz et al., 2017). Finally, a brief behavioral intervention was associated with a 22 percent increase in the proportion of patients who reported disposing of, or intent to dispose of, unused opioids in a pilot RCT involving cancer patients (n = 79), but this finding was nonsignificant (Maughan et al., 2016). The downstream effects of this education, such as effects on opioid misuse and opioid-related morbidity and mortality, are unknown.
In summary, studies evaluating the effectiveness of patient education about prescription opioids are generally lacking. However, evidence does indicate that patients lack information about opioids, suggesting the need for such education. Information about the risks and benefits of opioids and alternative strategies for managing pain is being provided by several organizations, but because these efforts have not been evaluated, their impact is unclear. Preliminary research suggests that patient education on safe storage and disposal of opioids is associated with self-reported improvements in measures of these outcomes.
Mass Media Campaign for General Public
In parallel with the committee's recommended changes to provider education and payer policy is the need to effect a major change in patient expectations in the treatment and management of chronic pain. The committee was struck particularly by the relative lack of attention to the impact of education of the general public (i.e., all potential patients) about the risks and benefits of opioid therapy and the comparative effectiveness of opioid and nonopioid analgesics and nonpharmacologic interventions. Therefore, the committee recommends that the nation's public health leadership, including the surgeon general, the U.S. Centers for Disease Control and Prevention, and heads of major foundations and professional organizations, convene a body of experts in communication and in pain and opioid use disorder to evaluate the likely impact (and cost) of an education program designed to raise awareness among patients with pain and the general public about the risks and benefits of prescription opioids and to promote safe and effective pain management (Recommendation 5-5).16
Increasing Access to and Utilization of Medical Treatment for Opioid Use Disorder
As discussed in Chapter 4, medication-assisted treatment (MAT) is the central component of evidence-based treatment for OUD, regardless of whether it is combined with behavioral therapy. The use of medication can help patients cope with withdrawal symptoms, and may relieve drug cravings without producing the “high” of opioids. The medications that are used in MAT are opioid agonists, partial agonists, or antagonists, and include methadone, buprenorphine, naltrexone, and combination buprenorphine-naltrexone (Suboxone®). Research is ongoing into new MAT drug products, including implantable and “vaccine”-type medications.
Delivery Models
Integrating buprenorphine maintenance therapy (BMT) into federally qualified health centers (FQHCs) has been shown to be feasible, to increase access to evidence-based treatment for OUD, to expand the scope of patient-centered medical homes (a model of primary care under the ACA that is patient-centered, comprehensive, accessible, and focused on quality), and to reduce illicit opioid use (Haddad et al., 2013). Integrating BMT into FQHCs also resulted in improved engagement of patients in primary care, preventive screening for other health conditions, and quality health care indicators beyond treatment of OUD. Additional strategies may be needed for women and those retained in treatment for less than 3 months, as they were less likely than their counterparts to receive preventive screening, which resulted in lower-quality health care indicator scores for these populations (Haddad et al., 2015).
An RCT comparing three approaches used in emergency department–initiated buprenorphine-naloxone treatment for OUD found that those who received screening, brief intervention, and referral to primary care for 10-week follow-up had superior outcomes relative to two comparison conditions (screening and treatment referral; and screening, brief intervention, and facilitated referral to community-based treatment services). Superior outcomes were noted for engagement in treatment 30 days postrandomization and reduced days of self-reported illicit opioid use per week. The rate of negative urine screens did not differ by study condition (D'Onofrio et al., 2015).
With regard to criminal justice settings, an RCT of prison-initiated buprenorphine treatment for inmates who were heroin-dependent prior to incarceration found significant effects favoring the buprenorphine treatment compared with counseling only (99 versus 80.4 percent) and for entry into treatment in a community setting compared with an opioid treatment center (47.5 versus 33.7 percent). Women were significantly more likely than men to complete treatment (85.7 versus 52.7 percent) (Gordon et al., 2014). A study of the impact of opioid treatment therapy in correctional settings in Australia found high treatment retention during incarceration (82 percent), prescriptions for MAT provided at release (90 percent), and presentation at community clinics for MAT postrelease (94 percent) (Larney et al., 2016).
State and Local Initiatives
Several state and local initiatives have been undertaken to increase access to and utilization of medical treatment for OUD. A buprenorphine initiative in Baltimore, Maryland, reduced opioid treatment waitlists and heroin overdose deaths by using a team of health care workers to support patients while they were in short-term treatment at a substance use disorder treatment facility, help them access Medicaid coverage, and refer them to outpatient providers for continuing care (Schwartz et al., 2013).
The Massachusetts Department of Public Health has implemented a nurse management model that encompasses initial assessment; referral to treatment; adherence monitoring; and communication with prescribing physicians, addiction counselors, and pharmacists. This model allows physicians with buprenorphine waivers to take on more patients (Alford et al., 2011). The expansion of this collaborative model for delivery of opioid agonist therapy with buprenorphine to 14 community health centers in Massachusetts led to a 375 percent increase in the number of waivered physicians (enabling their prescribing of buprenorphine) within 3 years (LaBelle et al., 2016).
Vermont's regional infrastructure for treatment of substance use disorder utilizes both geographic area–specific centers (“hubs”) to provide comprehensive services to individuals with OUD and teams of clinicians (“spokes”) to provide treatment, counseling, and other services to individuals who are less clinically complex. A cross-sectional study conducted during 2008 to 2013 evaluated outcomes for Vermont Medicaid beneficiaries with OUD, comparing those receiving MAT with those receiving treatment without medication. Results suggest that MAT is associated with reduced general health care expenditures and utilization, such as inpatient hospital admissions and outpatient emergency department visits. The costs of treatment therefore were offset by these savings (Mohlman et al., 2016).
Treatment Utilization
State Medicaid policies influence enrollees' access to and use of opioid agonists (e.g., methadone and buprenorphine) for treatment of OUD. Most states cover such treatment for Medicaid enrollees, and the number of enrollees covered increased from 2004 to 2013. However, some states do not cover both methadone and buprenorphine. Furthermore, obstacles to utilization of opioid agonists exist, such as prior authorization requirements; copayments; and requirements for concurrent counseling, which if not available can act as a barrier to the treatment (Burns et al., 2015). State policies regarding coverage of the treatment have been associated with an increase in buprenorphine-waivered physicians (Stein et al., 2015) and with use of opioid agonist therapies and buprenorphine in substance use disorder treatment facilities (Bauhoff et al., 2014; Ducharme and Abraham, 2008). Mark and colleagues (2015) found that while 12 percent of Medicaid recipients had substance use disorders, only 13 state Medicaid programs included all medications approved for treatment of alcohol and opioid substance use disorder on their preferred drug lists. The drugs that were most commonly excluded were ER naltrexone, acamprosate, and methadone. Forty-eight Medicaid programs required prior authorization for combined buprenorphine-naloxone treatment, and 11 had 1- to 3-year lifetime treatment limits (Mark et al., 2015).
Availability of Providers and Treatment
Insufficient numbers of providers for treatment of OUD have been noted as a significant barrier to the availability of such treatment. In a state-level analysis of the supply of physicians waivered to prescribe buprenorphine for OUD, Knudsen (2015) found that the average state had 8 waivered physicians per 100,000 residents. In addition, large regional differences were found between states in the Northeast and states in the Midwest, South, and West. The supply of physicians waivered to prescribe buprenorphine was positively associated with the percentage of residents covered by Medicaid, the population-adjusted availability of opioid treatment programs, and the number of substance use disorder treatment programs. The supply of waivered physicians was positively correlated with states' numbers of overdose deaths, suggesting that physicians may seek waivers in response to the level of the opioid problem in their state (Knudsen, 2015). Recent steps to expand the number of waivered providers include increasing the upper limit of patients that can be treated by waivered physicians, expanding the type of prescribers permitted to be DATA17waivered, and integrating the required training into the health care professional educational curriculum (ASAM, 2016). For instance, the state of Rhode Island has taken steps to expand access to OUD treatment by incorporating the required training into existing medical school curriculum (McCance-Katz et al., 2017).
Significant gaps exist between the need for MAT and capacity. Jones and colleagues (2015) report that in 2012, the national rate of opioid misuse or dependence was 891.8 per 100,000 people aged 12 or older, while the treatment capacity was 420.3 for buprenorphine and 119.89 for methadone. Forty-eight states and the District of Columbia had past-year opioid misuse or dependence rates higher than their buprenorphine treatment capacity. While states varied significantly in their treatment need and capacity gap, most states (77.6 percent) reported that at least 75 percent of their treatment programs were operating at 80 percent capacity or greater. Although capacity for MAT increased markedly between 2003 and 2012, driven largely by the increase in the number of waivered physicians, the large gap between treatment need and capacity did not close significantly. The authors call for national and state practice and policy strategies to increase treatment capacity, such as improving training of health care professionals in the diagnosis and treatment of addiction; removing insurance, administrative, and payment-related obstacles; raising the limit on the number of patients physicians can treat with buprenorphine; and expanding the types of providers who can prescribe buprenorphine under the Drug Addiction and Treatment Act (Jones et al., 2015).
Increases in the availability of methadone and buprenorphine treatment have been linked to decreases in overdose deaths (Schwartz et al., 2013). However, MAT has been adopted in fewer than half of private-sector treatment programs, and when offered, only about one-third of patients receive it (Knudsen et al., 2011). Volkow and colleagues (2014) note that contributors to low access to and utilization of treatment with medication include the paucity of trained providers; negative attitudes regarding this form of treatment among providers, patients, and the general public; policy and regulatory barriers, such as utilization management techniques that place limits on dosages; treatment length; cumbersome paperwork for authorization and reauthorization; and minimal counseling coverage.
Treatment-Related Disparities
Studies show disparities in access to and utilization of treatment for substance use disorder in general and OUD in particular by race, ethnicity, and income.
Data from the National Epidemiologic Survey on Alcohol and Related Conditions show that both U.S.-born and immigrant Hispanic people who use drugs are less likely than their non-Hispanic white counterparts to have used any type of substance use disorder treatment (Mancini et al., 2015). The relationship between nativity and utilization of substance use disorder services varied among Hispanic groups, with utilization by Puerto Ricans being higher among those born on the island of Puerto Rico relative to those born in the continental United States. The authors point to several documented barriers to substance use disorder treatment among Hispanics, such as family factors, insurance/costs, linguistic and cultural factors, and the fit of service need with existing programs. The lifetime prevalence of use of heroin (as well as other drugs) was greater among U.S.-born relative to immigrant Hispanics after controlling for confounders, a finding that corroborates those of previous studies (Mancini et al., 2015). Data from an urban sample of the Treatment Episode Data Set-Discharges, a national census of annual discharges from substance use disorder treatment facilities, indicate that Hispanics and blacks are less likely to complete outpatient treatment relative to their white counterparts. Among heroin users, Hispanics were only 75 percent as likely as whites to complete a treatment episode (Mennis and Stahler, 2016).
For OUD specifically, a study of geographic and demographic differentials in uptake of buprenorphine compared with methadone treatment in New York City neighborhoods between 2004 and 2013 found that buprenorphine treatment had increased in all social areas over time, but that increases had been significantly higher in areas with the highest income and lowest percentages of Hispanics, blacks, and low-income residents. Overall, methadone treatment had remained stable over time (Hansen et al., 2016). Another study (the RAPiDs study) examined variables affecting enrollment in treatment among Rhode Island young adult users of nonmedical prescription opioids. This study found that nonwhite race and low income, as well as previous incarceration and having experienced drug-related discrimination by medical providers, were associated with significantly lower rates of treatment enrollment (Liebling et al., 2016).
In an analysis of the demographic characteristics and behavioral health of persons aged 12 and older that met criteria for past-year OUD (n = 6,125) in the 2005–2013 National Surveys on Drug Use and Health, Wu and colleagues (2016) found that greater than 80 percent of those with OUD had another substance use disorder, and 28.7 percent had experienced a major depressive episode. Among persons with OUD, 26.2 percent had used any treatment for alcohol or drug use, and 19.4 percent had used opioid-specific treatment. Opioid-specific treatment was especially underutilized by adolescents, the uninsured, blacks, Native Hawaiians/Pacific Islanders/Asian Americans, persons with prescription OUD only, and persons without major depressive episodes or substance use disorder (Wu et al., 2016).
Individuals involved in the criminal justice system also face barriers to effective treatment. While these individuals have high rates of substance use disorder (60–80 percent), their treatment utilization is low. Examining data from the Arrestee Drug Abuse Monitoring II program, Hunt and colleagues (2015) found that those with a history of heroin use had higher drug use and severity and higher rates of treatment utilization than those reporting use of other drugs. However, a minority (34 percent) of arrestees with drug use histories had received substance use disorder treatment during their lifetime, and only 14 percent had obtained such treatment during the year prior to their arrest. Receipt of mental health treatment services also is extremely low in this population despite a high prevalence of mental health problems.
More than 53 percent of state prison and local jail inmates meet diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) for drug abuse or dependence, and 19 percent have a lifetime history of heroin use (Belenko et al., 2013). However, a low proportion of those who could benefit from treatment receive it. When treatment with medication is offered, it is typically limited to detoxification, and often is provided only to pregnant women. Moreover, about half of drug courts have a specific policy against use of treatment with medication. Yet studies have demonstrated the efficacy of treatment with medication (i.e., methadone, buprenorphine, injectable sustained-release naltrexone) in criminal justice populations. Lack of treatment uptake in the criminal justice system may reflect state and local regulations, security concerns, institutional philosophy, and availability and resources. Additional research is needed on strategies for how best to integrate treatment into the criminal justice system at all stages (Belenko et al., 2013).
Summary
MAT for OUD has been found to be effective in a number of delivery models and settings but is greatly underutilized. This underutilization is driven by a combination of factors that include policies related to insurance coverage, payment, and approval and reimbursement limitations; lack of availability of eligible providers; negative attitudes toward treatment with medication among providers, patients, and the general public; insufficient training in OUD and its treatment among medical providers; and disparities in access and utilization. Aside from its immediate benefits to individuals with OUD, a strategy of increasing access to and utilization of treatment for OUD can be expected to diminish the risk of public health harms in the broader community by lowering the number of individuals engaging in opioid misuse. State and local governments are well positioned to take responsibility for ensuring universal access to treatment of OUD, using whatever financial and technical assistance is available from the federal government. To enhance these benefits, additional research could examine several relevant areas, such as (1) development of new medications; (2) testing of the efficacy of combination drugs (e.g., combining buprenorphine and naloxone to decrease potential for misuse); testing of the efficacy of approaches for increasing utilization in various key treatment settings, reducing negative side effects (including those related to inappropriate opioid/benzodiazepine prescribing), and reducing disparities in utilization; (3) testing of the efficacy of therapies combining medication and behavioral treatment; and (4) testing of alternative pain management methods for reducing the iatrogenic effects of pain management on opioid addiction. See Chapter 3 for the committee's formal research recommendation.
The enormity of the current opioid crisis necessitates an immediate and massive expansion of treatment capacity to provide evidence-based treatment and recovery to millions of individuals. More than 2 million people have a prescription opioid–related OUD, and almost 600,000 have a heroin-related OUD (HHS, 2016). To address the gap between the availability of and demand for treatment, the committee recommends that states, with assistance from relevant federal agencies, particularly the Substance Abuse and Mental Health Services Administration, provide universal access to evidence-based treatment for opioid use disorder (OUD), including use of medication, in a variety of settings, including hospitals, criminal justice settings, and substance use treatment programs. Efforts to this end should be carried out with particular intensity in communities with a high burden of OUD. State licensing bodies should require training in treatment for OUD for all licensed substance use disorder treatment facilities and providers (Recommendation 5-6).
The committee recommends that schools for health professional education, professional societies, and state licensing boards require and provide basic training in the treatment of opioid use disorder for health care providers, including but not limited to physicians, nurses, pharmacists, dentists, physician assistants, psychologists, and social workers (Recommendation 5-7).
The committee recommends that the U.S. Department of Health and Human Services and state health financing agencies remove impediments to full coverage of medications approved by the U.S. Food and Drug Administration for treatment of opioid use disorder (Recommendation 5-8).
STRATEGIES FOR REDUCING HARM
Drug use can have a number of negative consequences, including lowered quality of life, transmission of disease through intravenous needles, and increased morbidity and mortality. Many of the tools of drug policy are aimed at reducing or ending the use of drugs. These tools utilize a variety of methods, including individual rehabilitation and treatment, enforcement of criminal sanctions against drug use or distribution, and public communication campaigns aimed at preventing drug use. The priority of the harm reduction approach, in contrast, is minimizing the negative consequences of drug use instead of focusing solely on reducing drug use itself. Harm reduction encompasses multiple strategies tailored to the needs of particular individuals and communities, and may focus on encouraging safer drug use, managed use, and/or abstinence.
Two of the most significant harms of opioid use are overdose and transmission of bloodborne infections due to injection drug use. As discussed in Chapter 4, opioid-related overdoses have soared in recent years; in 2015, more than 33,000 people died from opioid overdoses, nearly half of which involved a prescription opioid (Rudd et al., 2016). Harm reduction strategies for opioids are aimed primarily at these two harms. Strategies for reducing the harms of opioid use may include dispensing naloxone for use in reversing overdose, providing services that facilitate safer drug use (syringe exchange, supervised injection facilities, and drug checking), and implementing behavioral interventions. Changes in drug laws also can be effective (see Box 5-4 for an international example). Often, harm reduction strategies are implemented together (see Box 5-5 for an example). Thus, naloxone is provided along with training in how to use it, and syringe exchange facilities also facilitate treatment admission or other services, educate users about overdose prevention and abscess and wound care, and provide training in the use of naloxone.
Use of Naloxone to Reverse Overdose
As discussed in Chapter 4, naloxone is an opioid antagonist of the µ opioid receptor. When administered, it blocks the effects of opioids and reverses depression of the respiratory and central nervous systems, preventing death by overdose. Naloxone can be administered via intravenous, intramuscular, subcutaneous, or intranasal routes. In 2014, the FDA approved a naloxone autoinjector system that provides the administrator with voice and visual guidance, and in late 2015, the FDA approved a naloxone nasal spray, which is easy to administer and eliminates the risk of a contaminated needle stick. Naloxone is not a controlled substance and has no abuse potential, but when administered to people who are dependent on opioids, it may cause acute withdrawal symptoms, including vomiting.
BOX 5-4
Outcomes Associated with a Harm Reduction Strategy in Portugal.
Overdoses can occur among all groups of opioid users—those who use illicit opioids, those who misuse prescription opioids, and those who use opioids to manage pain as prescribed by a doctor. Naloxone training and distribution programs have historically been targeted at users of illicit opioids, particularly people who use drugs intravenously, because they are at high risk and are also most likely to report using the medication to reverse an overdose (Rowe et al., 2015). However, there is growing interest in translating these programs into clinical settings for patients who take prescription opioids (Mueller et al., 2015). Because anyone who uses opioids is at risk of overdose, various strategies are used to make naloxone available in a variety of settings. These strategies can be divided roughly into community-based, systems-based, pharmacy-based, and prescriber-based.
BOX 5-5
Harm Reduction Strategies in Huntington, West Virginia.
There are a number of barriers to the use of naloxone to prevent overdose. First is a simple logistical barrier: the person who is overdosing cannot self-administer naloxone, so there must be someone nearby who can recognize the symptoms of overdose, can quickly access naloxone, and knows how to administer it. There also are legal and regulatory barriers. For example, naloxone requires a prescription in some states, a nonmedical person who administers naloxone can face potential liability, and people who use drugs who summon aid for an overdose can face potential legal ramifications. Most states have passed laws to address these various barriers. New Mexico, for example, passed the first law protecting lay administrators of naloxone in 2001 and the first “Good Samaritan” law to protect users who summon help in 2007 (Network for Public Health Law, 2016). Dozens of states have followed suit. Rhode Island has made particular progress in eliminating the legal barriers to the use of naloxone (see Box 5-6). The adoption of these laws has been shown to be associated with a decrease in opioid-related deaths. Rees and colleagues (2017) examined the effect of naloxone access laws and Good Samaritan laws. They found that the adoption of a naloxone access law is associated with a 9–11 percent reduction in opioid-related deaths, while the adoption of a Good Samaritan law appears to be associated with a similar reduction, although this association is not statistically significant. The authors note that the naloxone access laws most strongly associated with a decrease in deaths are those that remove criminal liability for possession of naloxone (Rees et al., 2017).
Making changes to the legal landscape requires, of course, some level of public support for the changes, and the public does not always support the provision of naloxone, despite its obvious and immediate benefits (see Box 5-7 for a review of state laws regarding naloxone). Critics of naloxone programs argue that the availability of naloxone will encourage increased drug use because users will rely on it to save them from overdose, or that using naloxone is futile because people who overdose and are saved will only overdose again in the future. This latter example is supported by modeled evidence: overdose predicts subsequent overdose (Coffin and Sullivan, 2013). However, a similar argument could be made against the use of cardiac catheterization in people who have experienced myocardial infarction (MI) as a strategy to prevent future MI, the difference in this case being that the underlying obesity or other predictors of MI may be less stigmatized than opioid misuse or OUD. This is an important point, because the public's low level of knowledge about or familiarity with naloxone and lack of sympathy for people who use drugs impact the level of support for naloxone distribution (Bachhuber et al., 2015). However, one study showed that these perceptions could be changed through exposure to messaging, particularly that which included factual information along with a sympathetic narrative about an individual who could have been saved with naloxone (Bachhuber et al., 2015). The final barrier is cost. Demand for naloxone has risen dramatically as the opioid epidemic has worsened and as states have facilitated and promoted the lay use of naloxone. Companies recently have raised the price of naloxone; in one case, Kaleo Pharma raised the price for its specific pack of two single-dose injectors from $750 to $3,750 (Silverman, 2016). Lack of widespread insurance coverage further exacerbates the cost issues of naloxone, particularly for third-party prescriptions (currently legal in 44 jurisdictions; see Box 5-7).
BOX 5-6
Improved Access to Naloxone in Rhode Island.
BOX 5-7
State Laws on Naloxone.
Community-Based Programs
Overdose education and naloxone distribution programs are designed to train people in the community who are most likely to witness an overdose—people who use drugs and their friends and family. Training programs that provide information about recognizing and responding to an overdose have existed since the mid-1990s, but in recent years have increasingly focused on providing naloxone to trainees (CDC, 2012). The trainings are often offered in conjunction with other services aimed at people who use drugs, such as syringe exchange programs; as a result, the trainees tend to be largely users of illicit opioids (e.g., heroin), despite the fact that nearly half of opioid overdoses involve a prescription drug (Clark et al., 2014).
A 2014 systematic review of community-based overdose education and naloxone distribution programs found that they are effective at increasing bystander knowledge about recognizing and responding to an overdose, and that this increased knowledge results in the successful use of naloxone and a high survival rate among those treated (Clark et al., 2014). Among the studies that measured knowledge before and after the training, many found a statistically significant increase in knowledge, although retention of this knowledge was variable. The primary components of the training included information about recognizing and preventing overdose; risk factors for overdose; and appropriate response to overdose, including naloxone administration.
According to an analysis of 19 Massachusetts communities adopting overdose education and naloxone distribution programs, rescue with naloxone was attempted 327 times between September 2006 and December 2009. The reported survival rate of overdose victims was high—98 percent overall—and the authors suggest that these trainings were associated with reduced mortality from opioid overdose at the population level (Walley et al., 2013). In addition to information about naloxone, trainees in these programs often receive information about other appropriate responses to overdose, including placing the person in the “recovery position,” using cardiopulmonary resuscitation (CPR), and contacting emergency medical services (EMS). Yet while some studies report that training improved the use of appropriate responses, many trainees continued to use inappropriate responses (e.g., throwing water on the victim), and most did not contact EMS. The failure to contact EMS often was due to a fear of negative consequences, although those who did contact EMS generally reported positive experiences (Clark et al., 2014).
While many users of naloxone obtain the drug through a formal training program, one retrospective cohort study in Massachusetts suggests that people who obtain naloxone through other means (e.g., their social networks) can and do use it successfully to reverse overdoses. Nor do their responses to overdose differ significantly from those of people who have been trained in the provision of naloxone (Doe-Simkins et al., 2014).
Systems-Based Programs
Naloxone distribution and training can also be conducted through health systems such as the Veterans Health Administration (VHA). Veterans are at particular risk of opioid-related harms, as many suffer from chronic pain and take opioids to treat it. About 68,000 veterans—roughly 13 percent of the total population of veterans who take opioids—have OUD, and veterans are twice as likely as nonveterans to die from accidental opioid overdoses (Childress, 2016). To address these issues, the VHA launched its Opioid Safety Initiative in October 2013. This initiative has reduced the use of opioids among veterans while seeking to manage their pain in other ways, and monitors the VHA's opioid dispensing practices systemwide. The VHA also launched the Opioid Overdose Education and Naloxone Distribution program in May 2014 to reduce opioid-related morbidity and mortality. This program encourages VA providers to consider providing education and naloxone to veterans who are at risk of opioid overdose, and gives providers tools for identifying such veterans using such information as opioid dosage, history of overdose, and other substance use disorder (VA, 2016).
Pharmacy-Based Programs
Research has shown that pharmacists are in an excellent position to train patients and their families on the use of naloxone kits (Bachyrycz et al., 2016; Bailey and Wermeling, 2014; Green et al., 2015b), although availability of the kits is not universal, and attitudes toward their use currently vary (Nielsen et al., 2016). Many states allow pharmacists to distribute naloxone over the counter without a prescription from a doctor. As of December 2016, this was the case in 33 states and the District of Columbia, with plans to expand to 7 more states in 2017 (see Walgreens, 2016). Pharmacists' knowledge, training, and position of trust put them in an ideal position to provide naloxone and counsel patients in when and how to use it. In the course of their work, most pharmacists “likely [are] serving some people who are misusing” prescription or illicit opioids (see APhA, 2015), and “can serve as invaluable instruments in identifying high-risk patients” (Bailey and Wermeling, 2014). Pharmacists interact daily with patients who are filling prescriptions for opioid analgesics, and in states that permit over-the-counter sales of syringes, with people who inject drugs. Because pharmacies are spread throughout neighborhoods and visited frequently by community members, the provision of naloxone through pharmacists greatly expands its accessibility, potentially enabling it to reach communities that are not served by other naloxone distribution programs (Green et al., 2015b).
Provider-Based Programs
Health care providers have an important role to play in reducing the harms of opioid use, both for users of illicit opioids and for patients who use opioid analgesics. Health care professionals can identify patients who are at risk of OUD or overdose, and can prescribe naloxone for patients who are taking opioids. Coprescription of opioids and naloxone is a fairly new practice, but some research suggests that it is well received by patients and can actually result in safer opioid use behaviors. Phillip Coffin, who oversees a project in which California clinics prescribe naloxone to any chronic pain patient who has used opioids for more than 3 months, says he is “looking for a change in the way that people interact with their opioid. The naloxone is there and will hopefully never be used, but I hope it helps people recognize the real risk of prescription opioids” (Alcorn, 2014). A nonrandomized study of such clinics compared those patients who were and were not prescribed naloxone along with their opioid prescription. Patients who had previously had an opioid-related emergency department visit or who were prescribed higher doses of opioids were more likely to be offered naloxone. Compared with patients who did not receive a naloxone prescription, those who did had 63 percent fewer opioid-related emergency department visits after 1 year. Among those who were prescribed naloxone, 82 percent filled the prescription successfully, and 37 percent reported safer opioid use behaviors after receiving the prescription. Patients generally had a favorable opinion of naloxone: 97 percent said they believed that patients who are prescribed opioids for pain should also be offered naloxone, and 79 percent had either a positive or neutral response to being offered naloxone (Behar et al., 2016; Coffin et al., 2016). While this study was observational and may not be generalizable to other settings, it suggests that coprescription of naloxone is acceptable and may have additional benefits.
Coe and Walsh (2015) argue that while providing naloxone to all prescription opioid users is “probably unnecessary and perhaps not practicable,” providers should consider making it available to patients who are at high risk, including those who
- have a diagnosis of alcohol or drug use disorder;
- maintain on a high dose of opioids;
- are initiating or receiving methadone;
- use other prescription medications, particularly benzodiazepines;
- have comorbid psychiatric disorders and are at greater risk for suicide by overdose; and
- have cognitive impairments that could lead to accidental overingestion.
The CDC guideline for prescribing opioids recommends naloxone coprescription in similar cases, with an additional recommendation for those patients who are at risk of returning to high doses and who are no longer tolerant (e.g., patients recently released from prison) (Dowell et al., 2016).
Despite the benefits of coprescription of naloxone, however there are significant barriers to this strategy. Providers may lack knowledge about naloxone and its use to prevent overdose, may be unaware that their patients are at risk for overdose, or may be hesitant to prescribe naloxone for fear that patients will be offended or will treat naloxone as a safety net and take more risks with opioids (Binswanger et al., 2015). One qualitative study of primary care staff who prescribed opioids to patients revealed that many staff had significant gaps in their knowledge about naloxone and were uncertain as to which patients were at risk of overdose. The staff in the survey suggested that naloxone prescribing could be facilitated through standardized guidelines for prescribing, efforts to reduce the stigma of naloxone, and improved communication from emergency departments about overdoses and guidance for follow-up (Binswanger et al., 2015).
Patients who are at risk for overdose due to illicit drugs face an even greater barrier to obtaining naloxone or other harm reduction medications from their physicians. One study showed that 54 percent of physicians “would never consider prescribing naloxone to a patient who injected drugs” because of discomfort, lack of knowledge, or a belief that providing naloxone may condone risky drug use (Mueller et al., 2015). Health care professionals are in a prime position to identify and assist patients who are at risk for overdose, but stigma reduction efforts, education, and training are needed to capitalize on this opportunity.
Summary
Naloxone is a safe and effective method for reversing overdoses, and can easily be administered by bystanders. However, a number of barriers prevent it from being as widely used as it could be. These barriers include laws that do not allow community members to access naloxone or pharmacists to distribute it, its rising cost, and a lack of knowledge about it among health care providers. The committee recommends that state medical and pharmacy boards educate and train their members in recognizing and counseling patients who are at risk for opioid use disorder and/or overdose, and encourage providers and pharmacists to offer naloxone when an opioid is prescribed to these patients or when a patient seeks treatment for overdose or other opioid-related issues (Recommendation 5-9).
Reducing Disease Transmission
Syringe Exchange
Sharing syringes and drug injection equipment puts people who inject drugs at high risk of being infected with HIV and HCV, as well as hepatitis B virus. Unsafe drug use is responsible for about 8 percent of new HIV infections in the United States and has contributed to a recent 150 percent increase in HCV infections (CDC, 2015). Because such infections as HIV and HCV also can be spread through sexual activity or from mother to baby, reducing infections among people who inject drugs can help protect the whole community (CDC, 2015). Syringe exchange programs, whether in a community setting or through pharmacies, have proven an effective method for reducing the risk of infection. In addition to providing clean injection equipment, these programs can facilitate a number of other services that are useful for people who use drugs, including helping them find treatment options, HIV testing and counseling, access to naloxone, and education about safer injection practices and safer drug use. Because syringe exchange programs often are just one of a broader set of harm reduction interventions, it is difficult to determine the extent to which they reduce the risk of infection for people who inject drugs. Research does suggest, however, that syringe exchange programs are an effective strategy for reducing HCV seroconversion (Hagan et al., 2011) and are effective at encouraging and facilitating entry into drug treatment (SAMHSA, 2011). In late 2016, the CDC called on state and local health departments to improve access to syringe exchange, citing a CDC report noting that only one in four people who use injection drugs always use sterile injection equipment (Abbasi, 2017). Additionally, a CDC brief cites multiple studies demonstrating the cost savings resulting from legalized syringe exchange programs, primarily through reducing the prevalence of HIV, HCV, and related health care costs (CDC, 2016a).
In some communities, safe injection equipment is available directly from pharmacies. The sale of syringes through pharmacies is regulated by a patchwork of laws and regulations, including state laws that require a prescription for syringes and state drug paraphernalia laws that forbid the sale of items intended to be used to consume illegal drugs (see Box 5-8 for a summary of state laws regulating the possession or distribution of injection equipment).18However, some states have taken steps to improve access to clean syringes by exempting syringes from such laws. The American Pharmacists Association is supportive of these efforts; it “encourages state legislatures and boards of pharmacy to revise laws and regulations to permit the unrestricted sale or distribution of sterile syringes and needles by or with the knowledge of a pharmacist in an effort to decrease the transmission of blood-borne diseases” (APhA, 1999).
Making syringes available from pharmacies has great potential to expand the geographic reach of access to clean syringes (Logan and Deutsch, 2015). Pharmacists also can counsel users and facilitate other services; in fact, a 2015 California law mandates that pharmacies selling nonprescription syringes provide written or verbal counseling at the time of sale about accessing drug treatment, accessing HIV and HCV testing and treatment, and safely disposing of used injection equipment.19
BOX 5-8
Laws Concerning Injection Equipment.
Supervised Injection Facilities
Supervised injection facilities (SIFs) provide users a safe space to inject drugs (that are obtained elsewhere) under clinical supervision. The facilities often offer clean injection equipment; information about safer drug use; referrals for medical care, testing, and treatment; and other services (We are the Drug Policy Alliance, 2017). Research has shown that SIFs are associated with safer injection practices and higher uptake of treatment services (Beletsky et al., 2008). In addition to the benefits for people who use drugs, SIFs reduce drug-related public nuisances, such as public drug use and discarded syringes (Beletsky et al., 2008). There are more than 100 SIFs operating in 11 countries worldwide, but none in the United States (ScienceDaily, 2016). Efforts are under way to implement SIF pilot projects in the United States; a 2016 study estimated that a single SIF in San Francisco could generate $3.5 million in health savings per year (ScienceDaily, 2016). The city of Ithaca, New York, has developed a comprehensive drug plan that calls for the exploration of a SIF. The plan explains that a SIF could “prevent fatal and non-fatal overdose, infectious disease, and bacterial infections; reduce public drug use and discarded needles; and provide primary care and referrals to basic services, housing, and substance use services and treatment” (City of Ithaca, 2016, p. 7). In light of these initiatives, it appears likely that severely affected localities will seek to establish SIFs. If they do so, however, legal questions may arise about whether states or local governments could authorize the facilities and operate them without violating federal law. Such facilities could be established on an experimental basis for the purpose of estimating the effectiveness and cost of such programs.
Drug Checking
The heroin that the individuals in Huntington, West Virginia, had injected, as described earlier in Box 5-5, was found to be mixed with fentanyl (an opioid 50–100 times stronger than morphine) and carfentanil (an opioid used for tranquilizing elephants that is 10,000 times stronger than morphine) (Joseph, 2016). Drug checking services are designed to avert these kinds of tragedies by analyzing the purity of drugs and identifying the presence of adulterants; in addition, the services use this information to monitor the drug market and identify new or lethal drugs. Drug checking services have existed in Europe for several decades but are scarcer in the United States, consisting of only a handful of online services that test anonymously sent drug samples or provide at-home test kits (Johnson, 2016).
Behavioral Interventions
The medications and services discussed above often are offered in tandem with behavioral interventions, although the latter interventions may also be offered solo. Evidence suggests that behavioral interventions—such as trainings, education about safe injection practices, and motivational counseling—can result in increased knowledge, safer and/or reduced drug use, and lower risk of overdose or transmission of disease. Research has shown, for example, that opioid overdose training that includes information about how to recognize an overdose and administer naloxone significantly increases knowledge and confidence in administration (Ashrafioun et al., 2016).
Behavioral interventions can be delivered in a variety of settings, including the community, syringe exchange facilities, clinics, and pharmacies. One particularly promising setting for such interventions is the emergency department. People who seek help at an emergency department for opioid-related issues, including overdose, are in a prime position to be receptive to behavioral interventions, including education and treatment. Intervention in the emergency department is a fairly new strategy, so data on its effectiveness are limited, but early research suggests that this strategy can result in long-term behavior changes. A program begun in August 2014, for example, targets patients presenting with an opioid overdose in Rhode Island hospitals. Patients in the emergency department are given a naloxone kit and overdose prevention education, and are paired with a peer recovery coach who offers support and referral to addiction treatment (Samuels et al., 2014). The coaches are trained and certified by the Anchor Recovery Community Center, a peer-to-peer recovery support organization. Since the program's inception, 82 percent of people who have overdosed and been seen in Rhode Island hospitals have accepted a recovery coach, and 87 percent of them have remained engaged at the 30-day mark. Six months after their emergency department visit, 33 percent were still engaged and on the path to recovery (Goyer, 2016). Other emerging models for these types of interventions include the following:
- Safe Stations (Manchester, New Hampshire)—Fire stations are designated safe spaces for individuals who are seeking assistance on a path to recovery. Such individuals who arrive at fire stations are asked to dispose of needles, paraphernalia, and illegal substances, and then are medically assessed and may speak with recovery coaches and obtain further information about treatment.20
- Angel Program (Gloucester, Massachusetts): This program allows individuals to turn in their drugs to the police (without threat of arrest) and assigns them an “angel” to guide them through recovery. Early numbers suggest that the program saves money and may facilitate recovery. Of 100 program participants who answered a survey question, 60 had not returned to using drugs. Similar programs have begun in Chicago and North Carolina (Hasan, 2016).
Summary
Harm reduction strategies such as syringe exchanges, SIFs, and drug checking can not only facilitate safer drug use practices but also serve as a conduit for users to access treatment, medical care, and basic services. Unfortunately, while some strategies have been shown to reduce morbidity and mortality among people who use prescription and/or illicit opioids, there are significant barriers to access to safe injection equipment, most notably state laws.
To reduce the harms of opioid use, including death by overdose and transmission of infectious diseases, the committee recommends that states implement laws and policies that remove barriers to access to naloxone and safe injection equipment by
- permitting providers and pharmacists to prescribe, dispense, or distribute naloxone to laypersons, third parties, and first responders and by standing order or other mechanism;
- ensuring immunity from civil liability or criminal prosecution for prescribers for prescribing, dispensing, or distributing naloxone, and for laypersons for possessing or administering naloxone; and
- permitting the sale or distribution of syringes, exempting syringes from laws that prohibit the sale or distribution of drug paraphernalia, and explicitly authorizing syringe exchange (Recommendation 5-10).
SUMMARY AND RECOMMENDATIONS
Each of the above strategies involves costs and trade-offs. Every policy that aims to curtail opioid-related harms by reducing access to opioids (including reducing “overprescribing”) involves a potential therapeutic loss to patients in pain who have no satisfactory alternatives to opioids. The committee believes the restrictions, policies, and practices recommended in this report leave adequate space for responsible prescribing and reasonable access for patients and physicians who believe that an opioid is medically necessary. Another likely effect of restrictions on lawful access to prescription opioids is that some proportion of persons who have developed OUD will seek to satisfy their needs on the illicit market. One way of thinking about the policy trade-off is that curtailing access on the legal market to reduce the incidence of future iatrogenic OUD (and other harms) will drive persons who already have OUD to the illegal market. The committee regards the need to couple the long-run public health gain of reduced access with an investment in treatment for the millions of individuals with OUD as an ethical imperative.
Strategies for Restricting Supply
Recommendation 5-1. Improve access to drug take-back programs. States should convene a public–private partnership to implement drug take-back programs allowing individuals to return drugs to any pharmacy on any day of the year, rather than relying on occasional take-back events.
Strategies for Influencing Prescribing Practices
Recommendation 5-2. Establish comprehensive pain education materials and curricula for health care providers. State medical schools and other health professional schools should coordinate with their state licensing boards for health professionals (e.g., physicians, nurses, dentists, pharmacists), the National Institutes of Health's Pain Consortium, the U.S. Food and Drug Administration, the U.S. Centers for Disease Control and Prevention, and the U.S. Drug Enforcement Administration to develop an evidence-based national approach to pain education encompassing pharmacologic and nonpharmacologic treatments and educational materials on opioid prescribing.Recommendation 5-3. Facilitate reimbursement for comprehensive pain management. Public and private payers should develop reimbursement models that support evidence-based and cost-effective comprehensive pain management encompassing both pharmacologic and nonpharmacologic treatment modalities.Recommendation 5-4. Improve the use of prescription drug monitoring program data for surveillance and intervention. The U.S. Department of Health and Human Services, in concert with state organizations that administer prescription drug monitoring programs, should conduct or sponsor research on how data from these programs can best be leveraged for patient safety (e.g., data on drug–drug interactions), for surveillance of policy and other interventions focused on controlled substances (e.g., data on trends in opioid prescribing, effects of prescriber guidelines), for health service planning (e.g., data on discrepancies in dispensing of medications for treatment of opioid use disorder), and for use in clinical care (i.e., in clinical decision making and patient–provider communication).
Strategies for Reducing Demand
Recommendation 5-5. Evaluate the impact of patient and public education about opioids on promoting safe and effective pain management. The nation's public health leadership, including the surgeon general, the U.S. Centers for Disease Control and Prevention, and heads of major foundations and professional organizations, should convene a body of experts in communication and in pain and opioid use disorder to evaluate the likely impact (and cost) of an education program designed to raise awareness among patients with pain and the general public about the risks and benefits of prescription opioids and to promote safe and effective pain management.Recommendation 5-6. Expand treatment for opioid use disorder. States, with assistance from relevant federal agencies, particularly the Substance Abuse and Mental Health Services Administration, should provide universal access to evidence-based treatment for opioid use disorder (OUD), including use of medication, in a variety of settings, including hospitals, criminal justice settings, and substance use treatment programs. Efforts to this end should be carried out with particular intensity in communities with a high burden of OUD. State licensing bodies should require training in treatment for OUD for all licensed substance use disorder treatment facilities and providers.Recommendation 5-7. Improve education in treatment of opioid use disorder for health care providers.Schools for health professional education, professional societies, and state licensing boards should require and provide basic training in the treatment of opioid use disorder for health care providers, including but not limited to physicians, nurses, pharmacists, dentists, physician assistants, psychologists, and social workers.Recommendation 5-8. Remove barriers to coverage of approved medications for treatment of opioid use disorder. The U.S. Department of Health and Human Services and state health financing agencies should remove impediments to full coverage of medications approved by the U.S. Food and Drug Administration for treatment of opioid use disorder.
Strategies for Reducing Harm
Recommendation 5-9. Leverage prescribers and pharmacists to help address opioid use disorder. State medical and pharmacy boards should educate and train their members in recognizing and counseling patients who are at risk for opioid use disorder and/or overdose, and encourage providers and pharmacists to offer naloxone when an opioid is prescribed to these patients or when a patient seeks treatment for overdose or other opioid-related issues.Recommendation 5-10. Improve access to naloxone and safe injection equipment. To reduce the harms of opioid use, including death by overdose and transmission of infectious diseases, states should implement laws and policies that remove barriers to access to naloxone and safe injection equipment by
- permitting providers and pharmacists to prescribe, dispense, or distribute naloxone to laypersons, third parties, and first responders and by standing order or other mechanism;
- ensuring immunity from civil liability or criminal prosecution for prescribers for prescribing, dispensing, or distributing naloxone, and for laypersons for possessing or administering naloxone; and
- permitting the sale or distribution of syringes, exempting syringes from laws that prohibit the sale or distribution of drug paraphernalia, and explicitly authorizing syringe exchange.
REFERENCES
- AAFP (American Academy of Family Physicians). Chronic pain management and opioid misuse: A public health concern (position paper). 2017. [March 21, 2017]. http://www
.aafp.org/about /policies/all/pain-management-opioid.html. - Abbasi J. CDC says more needle exchange programs needed to prevent HIV. Journal of the American Medical Association. 2017;317(4):350. [PubMed]
- Afsharimani B, Kindl K, Good P, Hardy J. Pharmacological options for the management of refractory cancer pain: What is the evidence? Supportive Care in Cancer. 2015;23(5):1473–1481. [PubMed]
- Alcorn T. America embraces treatment for opioid drug overdose. Lancet. 2014;383(9933):1957–1958.[PubMed]
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Five year experience with collaborative care of opioid addicted patients using buprenorphine in primary care. Archives of Internal Medicine. 2011;171(5):425–431. [PMC free article] [PubMed]
- Alpert A, Powell D, Pacula AL. Supply-side drug policy in the presence of substitutes: Evidence from the introduction of abuse-deterrent opioids. Cambridge, MA: National Bureau of Economic Research; 2017. Working Paper 23031.
- Anderson DR, Zlateva I, Coman EN, Khatri K, Tian T, Kerns RD. Improving pain care through implementation of the Stepped Care Model at a multisite community health center. Journal of Pain Research. 2016;9:1021–1029. [PMC free article] [PubMed]
- AoME (Academy of Medical Educators). Professional standards. 2017. [March 21, 2017]. http://www
.medicaleducators .org/Professional-Standards. - APhA (American Pharmacists Association). APhA policy manual: Sale of sterile syringes. 1999. [January 7, 2017]. http://www
.pharmacist .com/policy-manual?page =11&key=pharmacist%20. - APhA. Old drug, new life: Naloxone access expands to community pharmacies. 2015. [April 17, 2017].http://www
.pharmacist .com/old-drug-new-life-naloxone-access-expands-community-pharmacies. - Arlotta CJ. The FDA's support for abuse-deterrent opioids may not be enough. Forbes. 2016 March 29; [April 26, 2017]; https://www
.forbes.com /sites/cjarlotta/2016 /03/29/the-fdas-support-for-abuse-deterrent-opioids /#6a872fe64ad6. - ASAM (American Society of Addiction Medicine). Summary of the Comprehensive Addiction and Recovery Act. 2016. [April 2, 2017]. http://www
.asam.org/advocacy /issues/opioids /summary-of-the-comprehensive-addiction-and-recovery-act. - ASHP (American Society of Health-System Pharmacists). ASHP guidelines on pharmacist-conducted patient education and counseling. 2011. [May 29, 2017]. https://www
.ashp.org /-/media/assets/policy-guidelines /docs/guidelines-pharmacist-conducted-patient-education-counseling .ashx?la=en. - Ashrafioun L, Gamble S, Herrmann M, Baciewicz G. Evaluation of knowledge and confidence following opioid overdose prevention training: A comparison of types of training participants and naloxone administration methods. Substance Abuse. 2016;37(1):76–81. [PubMed]
- Bachhuber MA, McGinty EE, Kennedy-Hendricks A, Niederdeppe J, Barry CL. Messaging to increase public support for naloxone distribution policies in the United States: Results from a randomized survey experiment. PLoS One. 2015;10(7):e0130050. [PMC free article] [PubMed]
- Bachyrycz A, Shrestha S, Bleske BE, Tinker D, Bakhireva LN. Opioid overdose prevention through pharmacy-based naloxone prescription program: Innovations in health care delivery. Substance Abuse. 2016;38(1):55–60.[PMC free article] [PubMed]
- Bailey AM, Wermeling DP. Naloxone for opioid overdose prevention: Pharmacists' role in community-based practice settings. Annals of Pharmacotherapy. 2014;48(5):601–606. [PMC free article] [PubMed]
- Barber C, Gagnon D, Fonda J, Hermos J, Miller M. Assessing the impact of prescribing directives on opioid prescribing practices among Veterans Health Administration providers. Pharmacoepidemiology and Drug Safety. 2017;26(1):40–46. [PubMed]
- Bauhoff S, Stein BD, Pacula RL. Do substance abuse policies influence opioid agonist therapies in substance abuse treatment facilities? Boston, MA: 2014. Paper presented at the Addiction Health Services Research Conference.
- Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: The impact on provider practices and overdose deaths, a systematic review. Journal of Opioid Management. 2016;12(2):109–118. [PubMed]
- Behar E, Rowe C, Santos GM, Murphy S, Coffin O. Primary care patient experience with naloxone prescription. Annals of Family Medicine. 2016;14(5):431–436. [PMC free article] [PubMed]
- Belenko S, Hiller M, Hamilton L. Treating substance use disorders in the criminal justice system. Current Psychiatry Reports. 2013;15(11):414. [PMC free article] [PubMed]
- Beletsky L, Davis CS, Anderson E, Burris S. The law (and politics) of safe injection facilities in the United States. American Journal of Public Health. 2008;98(2):231–237. [PMC free article] [PubMed]
- Bertsimas D, Patterson SS. The air traffic flow management problem with en route capacities. Operations Research. 1998;46(3):406–422.
- Binswanger IA, Glanz JM. Pharmaceutical opioids in the home and youth: Implications for adult medical practice. Substance Abuse. 2015;36(2):141–143. [PMC free article] [PubMed]
- Binswanger IA, Koester S, Mueller SR, Gardner EM, Goddard K, Glanz JM. Overdose education and naloxone for patients prescribed opioids in primary care: A qualitative study of primary care staff. Journal of General Internal Medicine. 2015;30(12):1837–1844. [PMC free article] [PubMed]
- Bird SM, Parmar MKB, Strang J. Take-home naloxone to prevent fatalities from opiate-overdose: Protocol for Scotland's public health policy evaluation, and a new measure to assess impact. Drugs: Education Prevention & Policy. 2015;22(1):66–76. [PMC free article] [PubMed]
- Bjørndal T, Lane DE, Weintraub A. Operational research models and the management of fisheries and aquaculture: A review. European Journal of Operational Research. 2004;156(3):533–540.
- Brandeis PMP COE (Prescription Monitoring Program Center of Excellence). Prescription monitoring programs: An effective tool in curbing the prescription drug abuse epidemic. 2012. [April 17, 2017].https://www
.bja.gov/publications /brandeis _pmp_effectiveness_brief.pdf. - Brandeis PMP COE. Using PDMPs to improve medical care: Washington State's data sharing initiative with Medicaid and workers' compensation. 2013. [December 6, 2016]. http://www
.pdmpassist .org/pdf/COE_documents /Add_to_TTAC/washington_nff_final.pdf. - Brandeis PMP COE. Using PDMP data to guide interventions with possible at-risk prescribers. 2014. [December 6, 2016]. http://www
.pdmpassist .org/pdf/COE_documents /Add_to_TTAC/Using _PDMP_Data_Guide_Interventions _at_Risk_Prescribers.pdf. - Brandeis PMP COE. Use of PDMP data by opioid addiction treatment programs. 2015. [December 6, 2016].http://www
.pdmpassist .org/pdf/COE_documents /Add_to_TTAC/Use%20of %20PDMP%20data%20by %20opioid%20treatment%20programs.pdf. - Brandeis PDMP TTAC (Prescription Drug Monitoring Program Training and Technical Assistance).Prescription drug monitoring frequently asked questions. 2017. [February 1, 2017]. http://www
.pdmpassist .org/content/prescription-drug-monitoring-frequently-asked-questions-faq. - Burns RM, Pacula RL, Bauhoff S, Hendrikson H, Leslie DL, Stein BD. Policies related to opioid agonist therapy for opioid use disorders: The evolution of state policies from 2004 to 2013. Substance Abuse. 2015;37(1):63–69. [PMC free article] [PubMed]
- Bushak L. Portugal's drug experiment: Tackling heroin addiction by decriminalizing drugs and focusing on health. Medical Daily. 2016 April 21; [March 1, 2017]; http://www
.medicaldaily .com/portugal-drug-experiment-heroin-decriminalizing-drugs-382598. - Campanella PE, Lovato E, Marone C, Fallacara L, Mancuso A, Ricciardi W, Speccia ML. The impact of electronic health records on healthcare quality: A systematic review and meta-analysis. European Journal of Public Health. 2016;26(1):60–64. [PubMed]
- Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Medicine. 2014;15(3):440–451.[PubMed]
- Caulkins JP, Kilmer B, Reuter PH, Midgette G. Cocaine's fall and marijuana's rise: Questions and insights based on new estimates of consumption and expenditures in U.S. drug markets. Addiction. 2014;110(5):728–736. [PubMed]
- Caulkins JP, Disley E, Tzvetkova M, Pardal M, Shah H, Zhang X. Modeling the structure and operation of drug supply chains: The case of cocaine and heroin in Italy and Slovenia. International Journal of Drug Policy. 2016;31:64–73. [PubMed]
- CDC (U.S. Centers for Disease Control and Prevention). Community-based opioid overdose prevention programs providing naloxone—United States, 2010. Morbidity and Mortality Weekly Report. 2012;61(6):101–105. [November 12, 2016]; https://www
.cdc.gov/mmwr/pdf/wk/mm6106 .pdf. [PMC free article] [PubMed] - CDC. CDC statement on syringe services programs—December 21, 2015. 2015. [January 5, 2017].https://www
.cdc.gov/nchhstp /newsroom/2015 /syringe_service_statement.html. - CDC. Access to clean syringes. 2016a. [May 22, 2017]. https://www
.cdc.gov/policy /hst/hi5/cleansyringes/index.html. - CDC. Guideline information for patients. Safer, more effective pain management. 2016b. [February 17, 2017].https://www
.cdc.gov/drugoverdose /prescribing/patients.html. - Chang HY, Lyapustina T, Rutkow L, Daubresse M, Richey M, Faul M, Stuart EA, Alexander GC. Impact of prescription drug monitoring programs and pill mill laws on high-risk opioid prescribers: A comparative interrupted time series analysis. Drug and Alcohol Dependence. 2016;165:1–8. [PMC free article] [PubMed]
- Chen JH, Hom J, Richman I, Asch SM, Podchiyska T, Johansen NA. Effect of opioid prescribing guidelines in primary care. Medicine (Baltimore). 2016;95(35):e4760. [PMC free article] [PubMed]
- Childress S. Veterans face greater risks amid opioid crisis. PBS Frontline. 2016 March 28; [January 10, 2017];http://www
.pbs.org/wgbh /frontline/article /veterans-face-greater-risks-amid-opioid-crisis. - Cicero TJ, Ellis MS, Surrat HL. Effect of abuse-deterrent formulation of OxyContin. New England Journal of Medicine. 2012;367(2):187–189. [PubMed]
- Cigna. Chronic pain: Policy overview. 2016. [April 28, 2017]. https://www
.cigna.com /healthwellness/hw/medical-topics /chronic-pain-cpain. - The Ithaca plan: A public health and safety approach to drugs and drug policy. 2016. [June 22, 2017]. City of Ithaca. http://www
.cityofithaca .org/documentcenter/view/4224. - Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. Journal of Addiction Medicine. 2014;8(3):153–163. [PubMed]
- Clark C. Mathematical bioeconomics: The optimal management of renewable resources. 2nd ed. New York: John Wiley & Sons, Inc; 1990.
- Clark T, Eadie J, Kreiner P, Strickler G. Prescription drug monitoring programs: An assessment of the evidence for best practices. 2013. [December 6, 2016]. http://www
.pewtrusts .org/~/media/assets/0001 /pdmp_update_1312013.pdf. - Cochella S, Bateman K. Provider detailing: An intervention to decrease prescription opioid deaths in Utah. Pain Medicine. 2011;12(Suppl. 2):S73–S76. [PubMed]
- Coe MA, Walsh SL. Distribution of naloxone for overdose prevention to chronic pain patients. Preventive Medicine. 2015;80:41–43. [PMC free article] [PubMed]
- Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Annals of Internal Medicine. 2013;158(1):1–9. [PubMed]
- Coffin PO, Behar E, Rowe C, Santos GM, Coffa D, Bald M, Vittinghoff E. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Annals of Internal Medicine. 2016;165(4):245–252. [PMC free article] [PubMed]
- Coplan PM, Kale H, Sandstrom L, Landau C, Chilcoat HD. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiology and Drug Safety. 2013;22(12):1274–1282. [PMC free article] [PubMed]
- Coplan PM, Chilcoat HD, Butler SF, Sellers EM, Kadakia A, Harikrishnan V, Haddox JD, Dart RC. The effect of an abuse-deterrent opioid formulation (OxyContin) on opioid abuse-related outcomes in the postmarketing setting. Clinical Pharmacology and Therapeutics. 2016;100(3):275–286. [PMC free article] [PubMed]
- Craig BM, Strassels SA. Out-of-pocket prices of opioid analgesics in the United States, 1999-2004. Pain Medicine. 2010;11(2):240–247. [PMC free article] [PubMed]
- Cunningham JK, Callaghan RC, Liu LM. U.S. federal cocaine essential (“precursor”) chemical regulation impacts on U.S. cocaine availability: An intervention time-series analysis with temporal replication. Addiction. 2015;110(5):805–820. [PMC free article] [PubMed]
- Cunningham JK, Liu L, Callaghan RC. Essential/precursor chemicals and drug consumption: Impacts of U.S. sodium permanganate and Mexico pseudoephedrine controls on the numbers of U.S. cocaine and methamphetamine users. Addiction. 2016;111(11):1999–2009. [PubMed]
- Cyclamed. Annual report. 2014. [February 24, 2017]. http://www
.cyclamed.org /wp-content/uploads /2015/06/Rapport-annuel-v-anglaiseHD- .pdf. - Daughton CG. Cradle to cradle stewardship of drugs for minimizing their environmental disposition while promoting human health—rationale for and avenues toward a green pharmacy. Environmental Health Perspectives. 2003;111(5):757–774. [PMC free article] [PubMed]
- Daughton CG. Pharmaceuticals in the environment: Sources and their management. In: Petrović M, Barceló D, editors. Analysis, fate and removal of pharmaceuticals in the water cycle. Vol. 50. Amsterdam, The Netherlands: Elsevier Science; 2007. pp. 1–58. Ch. 1.
- Davis CS, Pierce M, Dasgupta N. Evolution and convergence of state laws governing controlled substance prescription monitoring programs, 1988–2011. American Journal of Public Health. 2014;104(8):1389–1395.[PMC free article] [PubMed]
- Davis CS, Zaller N, Green TC. Addressing the overdose epidemic requires timely access to data to guide interventions. Drug and Alcohol Review. 2015;35(4):383–386. [PubMed]
- de la Cruz M, Reddy A, Balankari V, Epner M, Frisbee-Hume S, Wu J, Liu D, Yennuraialingam S, Cantu H, Williams J, Bruera E. The impact of an educational program on patient practices of safe use, storage, and disposal of opioids at a comprehensive cancer center. Oncologist. 2017;22(1):115–121. [PMC free article] [PubMed]
- DEA (U.S. Drug Enforcement Administration). Letter to registrants. 2014. [November 15, 2016]. https://www
.deadiversion .usdoj.gov/drug_disposal /dear_registrant_disposal.pdf. - DEA. DEA announces largest-ever prescription drug operation. Press Release. Washington, DC: DEA; 2015a. [March 1, 2017]. https://www
.dea.gov/divisions /no/2015/no052015.shtml. - DEA. DEA'S Prescription Drug Take-Back Effort—A Big Success. DEA Press Release. Washington, DC: 2015b. [November 12, 2016]. https://www
.dea.gov/divisions /hq/2015/hq100115.shtml. - DEA. Counterfeit prescription pills containing fentanyls: A global threat. DEA Intelligence Brief. Washington, DC: U.S. Department of Justice; 2016a. [April 17, 2017]. https://www
.dea.gov/docs /Counterfeit%20Prescription%20Pills .pdf. - DEA. 2016 national drug threat assessment summary. 2016b. [April 23, 2017]. https://www
.dea.gov/resource-center /2016%20NDTA%20Summary .pdf. - del Portal DA, Healy ME, Satz WA, McNamara RM. Impact of an opioid prescribing guideline in the acute care setting. Journal of Emergency Medicine. 2016;50(1):21–27. [PubMed]
- Delcher C, Wagenaar AC, Goldberger BA, Cook RL, Maldonado-Molina MM. Abrupt decline in oxycodone-caused mortality after implementation of Florida's Prescription Drug Monitoring Program. Drug and Alcohol Dependence. 2015;150:63–68. [PubMed]
- Dobkin C, Nicosia N. The war on drugs: Methamphetamine, public health, and crime. The American Economic Review. 2009;99(1):324–349. [PMC free article] [PubMed]
- Doe-Simkins M, Quinn E, Xuan Z, Sorensen-Alawad A, Hackman H, Ozonoff A, Walley AY. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: A retrospective cohort study. BMC Public Health. 2014;14:297.[PMC free article] [PubMed]
- DOJ (U.S. Department of Justice). United States reaches $8 million settlement agreement with CVS for unlawful distribution of controlled substances. 2016. [March 21, 2017]. https://www
.justice.gov /usao-md/pr/united-states-reaches-8-million-settlement-agreement-cvs-unlawful-distribution-controlled. - D'Onofrio G, O'Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: A randomized clinical trial. Journal of the American Medical Association. 2015;313(16):1636–1644. [PMC free article] [PubMed]
- Doorenbos AZ, Gordon DB, Tauben D, Palisoc J, Drangsholt M, Lindhorst T, Danielson J, Spector J, Ballweg R, Vorvick L, Loeser JD. A blueprint of pain curriculum across prelicensure health sciences programs: One NIH Pain Consortium Center of Excellence in Pain Education (CoEPE) experience. The Journal of Pain. 2013;14(12):1533–1538. [PMC free article] [PubMed]
- Dowell D, Haegerich T, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. Journal of the American Medical Association. 2016;315(15):1624–1645. [PubMed]
- Du Pen SL, Du Pen AR, Polissar N, Hansberry J, Kraybill BM, Stillman M, Panke J, Everly R, Syrjala K. Implementing guidelines for cancer pain management: Results of a randomized controlled clinical trial. Journal of Clinical Oncology. 1999;17(1):361–370. [PubMed]
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Substance Abuse Treatment, Prevention, and Policy. 2008;3:17. [PMC free article] [PubMed]
- Egan KL, Gregory E, Sparks M, Wolfson M. From dispensed to disposed: Evaluating the effectiveness of disposal programs through a comparison with prescription drug monitoring program data. American Journal of Drug and Alcohol Dependence. 2017;43(1):69–77. [PubMed]
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). Preventing overdose deaths in Europe. Lisbon, Portugal: EMCDDA; 2015.
- Eyre E. Drug firms poured 780M painkillers into WV amid rise of overdoses. Charleston Gazette-Mail. 2016 December 17; [March 1, 2017]; http://www
.wvgazettemail .com/news-health /20161217/drug-firms-poured-780m-painkillers-into-wv-amid-rise-of-overdoses#sthash.Lyer58dK.dpuf. - FBM (Florida Board of Medicine). Standards for the use of controlled substances for the treatment of pain.2010. [February 25, 2017]. http://www
.painpolicy .wisc.edu/sites/www .painpolicy.wisc.edu /files/Florida%20Medical %20Board%20Regulations_1.pdf. - FDA (U.S. Food and Drug Administration). Abuse-deterrent opioids—evaluation and labeling: Guidance for industry. 2015a. [February 24, 2017]. https://www
.fda.gov/downloads /Drugs/Guidances/UCM334743.pdf. - FDA. History. 2015b. [March 21, 2017]. https://www
.fda.gov/AboutFDA /WhatWeDo/History. - Training and continuing education. FDA. 2016a. [March 21, 2017]. https://www
.fda.gov/Training. - FDA. Summary minutes of the Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee joint meeting May 3-4, 2016. 2016b. [April 21, 2017].https://www
.fda.gov/downloads /AdvisoryCommittees /CommitteesMeetingMaterials /Drugs /AnestheticAndAnalgesicDrugProductsAdvisoryCommittee /UCM509895.pdf. - FDA. What to ask your doctor before taking opioids. 2016c. [February 17, 2017]. http://www
.fda.gov/ForConsumers /ConsumerUpdates/ucm529517 .htm. - FDA. 2017 meeting materials, Drug Safety and Risk Management Advisory Committee. 2017a. [March 15, 2017]. https://www
.fda.gov/AdvisoryCommittees /CommitteesMeetingMaterials /Drugs/DrugSafetyandRiskManagementAdvisoryCommittee /ucm536632.htm. - FDA. Introduction for the FDA blueprint for prescriber education for extended-release and long-acting opioid analgesics. 2017b. [February 5, 2017]. https://www
.fda.gov/downloads /Drugs/DrugSafety /InformationbyDrugClass/UCM515636 .pdf. - Fleming E, Proescholdbell S, Sachdeva N, Alexandridis AA, Margolis L, Ransdell K. North Carolina's Operation Medicine Drop: Results from one of the nation's largest drug disposal programs. North Carolina Medical Journal. 2016;77(1):59–62. [PMC free article] [PubMed]
- FSMB (Federation of State Medical Boards). Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013. [January 10, 2017]. http://www
.fsmb.org/Media /Default/PDF/FSMB /Advocacy/pain_policy_july2013.pdf. - FSMB. Guidelines for the chronic use of opioid analgesics. 2017. [May 28, 2017]. https://www
.fsmb.org /Media/Default/PDF/Advocacy /Opioid%20Guidelines %20As%20Adopted %20April%202017_FINAL.pdf. - Garg RK, Turner JA, Bauer AM, Wickizer T, Sullivan MD, Franklin GM. Changes in opioid prescribing for Washington workers' compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. The Journal of Pain. 2013;14(12):1620–1628. [PubMed]
- Gau JM, Brooke EJ. An assessment of the impact of a multipronged approach to reducing problematic pain clinics in Florida. Journal of Drug Issues. 2016;47(2)
- GCOAT (Governor's Cabinet Opiate Action Team). Ohio guidelines for prescribing opioids for the treatment of chronic, non-terminal pain 80 mg of a morphine equivalent daily dose (MED) “trigger point.”. 2013. [February 25, 2017]. http://mha
.ohio.gov/Portals /0/assets/Initiatives /GCOAT/Guidelines-Chronic-Pain .pdf. - Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid–involved overdose deaths—27 states, 2013–2014. Morbidity and Mortality Weekly Report. 2016;65(33):837–843. [PubMed]
- Glassmeyer ST, Hinchey EK, Boehme SE, Daughton CG, Ruhoy IS, Conerly O, Daniels RL, Lauer L, McCarthy M, Nettesheim TG, Sykes K, Thompson VG. Disposal practices for unwanted residential medications in the United States. Environment International. 2009;35(3):566–572. [PubMed]
- Goldstein ST, Fangjun Z, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. International Journal of Epidemiology. 2005;34(6):1329–1339. [PubMed]
- Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O'Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: Prison outcomes and community treatment entry. Drug and Alcohol Dependence. 2014;142:33–40. [PMC free article] [PubMed]
- Goyer J. Washington, DC: Sep 22, 2016. Presentation to the Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse.
- Gray JA, Hagemeier NE. Prescription drug abuse and DEA-sanctioned drug take-back events: Characteristics and outcomes in rural Appalachia. Archives of Internal Medicine. 2012;172(15):1186–1187. [PubMed]
- Gray JA, Hagemeier N, Brooks B, Alamian A. Prescription disposal practices: A 2-year ecological study of drug drop box donations in Appalachia. American Journal of Public Health. 2015;105(9):e89–e94. [PMC free article] [PubMed]
- Green LW, Kreuter MW. Evidence hierarchies versus synergistic interventions. American Journal of Public Health. 2010;100(10):1824–1825. [PMC free article] [PubMed]
- Green TC, Zaller N, Rich J, Bowman S, Friedmann P. Revisiting Paulozzi et al.'s “Prescription drug monitoring programs and death rates from drug overdose.” Pain Medicine. 2011;12(6):982–985. [PubMed]
- Green TC, Bowman S, Davis CS, Friedmann P. Discrepancies in addressing overdose prevention through prescription drug monitoring programs. Drug Alcohol and Dependence. 2015a;153:355–358. [PubMed]
- Green TC, Dauria EF, Bratberg J, Davis CS, Walley AY. Orienting patients to greater opioid safety: Models of community pharmacy-based naloxone. Harm Reduction Journal. 2015b;12:25. [PMC free article] [PubMed]
- Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: Real-world substance abuse treatment outcomes. Drug and Alcohol Dependence. 2013;131(1-2):127–135. [PMC free article] [PubMed]
- Haddad MS, Zelenev A, Altice FL. Buprenorphine maintenance treatment retention improves nationally recommended preventive primary care screenings when integrated into urban federally qualified health centers. Journal of Urban Health. 2015;92(1):193–213. [PMC free article] [PubMed]
- Haegerich TM. Prescription drug monitoring programs and other state level strategies. Washington, DC: Sep 22, 2016. Presentation to the Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse. http://www
.nationalacademies .org/hmd/Activities /PublicHealth /AddressPrescriptionOpioidAbuse /2016-SEP-22 /Videos/Session%204 /19-haegerich-video.aspx. - Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don't know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug and Alcohol Dependence. 2014;145:34–47. [PubMed]
- Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. Journal of Infectious Diseases. 2011;204(1):74–83. [PMC free article] [PubMed]
- Hansen H, Siegel C, Wanderling J, DiRocco D. Buprenorphine and methadone treatment for opioid dependence by income, ethnicity and race of neighborhoods in New York City. Drug and Alcohol Dependence. 2016;164:14–21. [PMC free article] [PubMed]
- Harle CA, Cook RL, Kinsell HS, Harman JS. Opioid prescribing by physicians with and without electronic health records. Journal of Medical Systems. 2014;38(11):138. [PMC free article] [PubMed]
- Harris K, Curtis J, Larsen B, Calder S, Duffy K, Bown G, Hadley M, Tristani-Firouzi P. Opioid pain medication use after dermatologic surgery: A prospective observational study of 212 dermatologic surgery patients. JAMA Dermatology. 2013;149(3):317–321. [PubMed]
- Hasan S. One year later: Gloucester's opioid program inspires policy reform. NPQ: Nonprofit Quarterly. 2016 June 3; [April 17, 2017]; https:
//nonprofitquarterly .org/2016/06/03 /one-year-later-gloucesters-opioid-program-inspires-policy-reform. - Haynes A, Kleinschmidt K, Forrester MB, Young A. Trends in analgesic exposures reported to Texas Poison Centers following increased regulation of hydrocodone. Clinical Toxicology. 2016;54(5):434–440. [PubMed]
- HHS (U.S. Department of Health and Human Services). Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. (NSDUH Series H-51). HHS Publication SMA 16-4984.
- Hill CS. Government regulatory influences on opioid prescribing and their impact on the treatment of pain of nonmalignant origin. Journal of Pain and Symptom Management. 1996;11(5):287–298. [PubMed]
- Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Annals of Surgery. 2017;265(4):709–714. [PubMed]
- Holliday SM, Hayes C, Dunlop AJ, Morgan S, Tapley A, Henderson KM, van Driel ML, Holliday EG, Ball JI, Davey A, Spike NA, McArthur LA, Magin PJ. Does brief chronic pain management education change opioid prescribing rates? A pragmatic trial in Australian early-career general practitioners. Pain. 2017;158(2):278–288.[PubMed]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Archives of General Psychiatry. 2001;58(5):503–508. [PubMed]
- Hughes CE, Stevens A. What can we learn from the Portuguese decriminalization of illicit drugs? The British Journal of Criminology. 2010;50(6):999–1022.
- Humphries CA, Counsell DJ, Pediani RC, Close SL. Audit of opioid prescribing: The effect of hospital guidelines. Anaesthesia. 1997;52(8):745–749. [PubMed]
- Hunt E, Peters RH, Kremling J. Behavioral health treatment history among persons in the justice system: Findings from the arrestee drug abuse monitoring II program. Psychiatric Rehabilitation Journal. 2015;38(1):7–15. [PubMed]
- Inciardi JA, Surratt HL, Kurtz SP, Cicero TJ. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Medicine. 2007;8(2):171–183. [PMC free article] [PubMed]
- IOM (Institute of Medicine). Ending the tobacco problem: A blueprint for the nation. Washington, DC: The National Academies Press; 2007.
- IOM. Relieving pain in America: A blueprint for transforming prevention, care, education, and research.Washington, DC: The National Academies Press; 2011.
- IOM. Public health implications of raising the minimum age of legal access to tobacco products. Washington, DC: The National Academies Press; 2015.
- Jann M, Kennedy WK, Lopez G. Benzodiazepines: A major component in unintentional prescription drug overdoses with opioid analgesics. Journal of Pharmacy Practice. 2014;27(1):5–16. [PubMed]
- Johnson CK. Could drug checking have prevented Prince's overdose death? The Big Story. 2016 October 4; [January 7, 2017]; http://bigstory
.ap.org /article/df36569d11294d918572d3edf5428cbe /could-drug-checking-have-prevented-princes-overdose-death. - Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes—Florida, 2010–2012. Morbidity and Mortality Weekly Report. 2014;63(26):569–574. [PMC free article] [PubMed]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health. 2015;105(8):e55–e63. [PMC free article] [PubMed]
- Joseph A. 26 overdoses in just hours: Inside a community on the front lines of the opioid epidemic. STAT. 2016 August 22; [March 1, 2017]; https://www
.statnews .com/2016/08/22/heroin-huntington-west-virginia-overdoses/ - Kaplan EH, Craft DL, Wein LM. Emergency response to a smallpox attack: The case for mass vaccination. Proceedings of the National Academy of Sciences of the United States of America; 2002. pp. 10935–10940. [PMC free article] [PubMed]
- KBML (Kentucky Board of Medical Licensure). Guidelines for the use of controlled substances in pain treatment. 2003. [February 25, 2017]. http://www
.kentucky.com /latest-news/article40998984 .ece/BINARY /Kentucky%20Board%20of %20Medical%20Licensure's %20guidelines %20for%20the%20use%20of %20controlled%20substances %20in%20pain%20treatment. - Kennedy-Hendricks A, Gielen A, McDonald E, McGinty EE, Shields W, Barry CL. Medication sharing, storage, and disposal practices for opioid medications among U.S. adults. JAMA Internal Medicine. 2016;176(7):1027–1029. [PubMed]
- Kerlikowske G, Jones CM, Labelle RM, Condon TP. Prescription drug monitoring programs—lack of effectiveness or a call to action. Pain Medicine. 2011;12(5):687–689. [PubMed]
- Kim N, Matzon JL, Abboudi J, Jones C, Kirkpatrick W, Leinberry CF, Liss FE, Lutsky KF, Wang ML, Maltenfort M, Ilyas AM. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: Identifying consumption patterns and determining prescribing guidelines. Journal of Bone and Joint Surgery. American Volume. 2016;98(20):e89. [PubMed]
- Knudsen HK. The supply of physicians waivered to prescribe buprenorphine for opioid use disorders in the United States: A state-level analysis. Journal of Studies on Alcohol and Drugs. 2015;76(4):644–654. [PMC free article] [PubMed]
- Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. Journal of Addiction Medicine. 2011;5(1):21–27. [PMC free article] [PubMed]
- LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): Statewide implementation of the Massachusetts Collaborative Care Model in Community Health Centers. Journal of Substance Abuse Treatment. 2016;60:6–13. [PMC free article] [PubMed]
- Laqueur H. Uses and abuses of drug decriminalization in Portugal. Law & Social Inquiry. 2015;40(3):746–781.
- Larney S, Lai W, Dolan K, Zador D. Monitoring a prison opioid treatment program over a period of change to clinical governance arrangements, 2007–2013. Journal of Substance Abuse Treatment. 2016;70:58–63.[PubMed]
- Law AV, Sakharkar P, Zargarzadeh A, Bilvick BW, Hess K, Hata M, Mireles R, Ha C, Park TJ. Taking stock of medication wastage: Unused medications in U.S. households. Research in Social and Administrative Pharmacy. 2015;11(2015):571–578. [PubMed]
- Lerner AM. The abuse of paregoric in Detroit Michigan (1956–1965). Bulletin on Narcotics. 1966;3:13–19.
- Levy DT, Nikolayev L, Mumford E. Recent trends in smoking and the role of public policies: Results from the SimSmoke tobacco control policy simulation model. Addiction. 2005;100(10):1526–1536. [PubMed]
- Li G, Brady JE, Lang BH, Giglio J, Wunsch H, DiMaggio C. Prescription drug monitoring and drug overdose mortality. Injury Epidemiology. 2014;1(1):9. [PMC free article] [PubMed]
- Liebling EJ, Yedinak JL, Green TC, Hadland SE, Clark MA, Marshall BD. Access to substance use treatment among young adults who use prescription opioids nonmedically. Substance Abuse Treatment, Prevention, and Policy. 2016;11(1):38. [PMC free article] [PubMed]
- Loeffler G, Craig C. The effect of legal bans on poison control center contacts regarding “legal highs.” Addiction. 2013;108(7):1348–1349. [PubMed]
- Logan K, Deutsch S. Room for improvement in the New York State pharmacy-based syringe access program. Columbia Medical Review. 2015;1(1):40–50.
- Long D, Lee D, Johnson J, Gaier E, Kostiuk P. Modeling air traffic management technologies with a queuing network model of the national airspace system. 1999. [January 6, 2017]. https://pdfs
.semanticscholar .org/b9c8/3126afb91cc91887c02e8d540a07e8063565.pdf. - Lyapustina T, Rutkow L, Chang HY, Daubresse M, Ramji AF, Faul M, Stuart EA, Alexander GC. Effect of a “pill mill” law on opioid prescribing and utilization: The case of Texas. Drug and Alcohol Dependence. 2016;159:190–197. [PMC free article] [PubMed]
- Maeng DD, Snyder RC, Medico CJ, Mold WM, Maneval JE. Unused medications and disposal patterns at home: Findings from a Medicare patient survey and claims data. Journal of the American Pharmacists Association. 2016;56(1):41–46. [PubMed]
- Mai J, Franklin G, Tauben D. Guideline for prescribing opioids to treat pain in injured workers. Physical Medicine and Rehabilitation Clinics of North America. 2015;26(3):453–465. [PubMed]
- Mancini MA, Salas-Wright CP, Vaughn MG. Drug use and service utilization among Hispanics in the United States. Social Psychiatry and Psychiatric Epidemiology. 2015;50(11):1679–1689. [PubMed]
- Mark TL, Lubran R, McCance-Katz EF, Chalk M, Richardson J. Medicaid coverage of medications to treat alcohol and opioid dependence. Journal of Substance Abuse Treatment. 2015;55:1–5. [PubMed]
- Maughan BC, Hersh EV, Shofer FS, Wanner KJ, Archer E, Carraso LR, Rhodes KV. Unused opioid analgesics and drug disposal following outpatient dental surgery: A randomized controlled trial. Drug and Alcohol Dependence. 2016;168:328–334. [PubMed]
- MBC (Medical Board of California). Guidelines for prescribing controlled substances for pain. 2014. [February 25, 2017]. http://www
.mbc.ca.gov /licensees/prescribing/pain_guidelines .pdf. - McCance-Katz EF, George P, Scott NA, Dollase R, Tunkel AR, McDonald J. Access to treatment for opioid use disorders: Medical student preparation. American Journal on Addictions. 2017;26(4):316–318. [PubMed]
- McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addictive Behaviors. 2013;38(6):2230–2235. [PMC free article] [PubMed]
- McKetin R, Sutherland R, Bright DA, Norberg MM. A systematic review of methamphetamine precursor regulations. Addiction. 2011;106(11):1911–1924. [PubMed]
- McLaughlin K. Oregon health plan opens door to back-pain treatment. 2016. [May 22, 2017]. http://www
.bendbulletin .com/home/3363729-151 /oregon-health-plan-opens-door-to-back-pain-treatment. - Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O'Malley AJ, Morden NE. State legal restrictions and prescription-opioid use among disabled adults. New England Journal of Medicine. 2016;375(1):44–53. [PMC free article] [PubMed]
- Mearis M, Shega JW, Knoebel RW. Does adherence to National Comprehensive Cancer Network guidelines improve pain-related outcomes? An evaluation of inpatient cancer pain management at an academic medical center. Journal of Pain and Symptom Management. 2014;48(3):451–458. [PubMed]
- Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science. 2009;325(5948):1705–1708.[PubMed]
- Megrey BA. A review and comparison of age-structured stock assessment models from theoretical and applied points of view. Seattle, WA: U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Northwest and Alaska Fisheries Center; 1988. [April 17, 2017]. https://docs
.lib.noaa .gov/noaa_documents /NMFS/AFSC/NWAFC_processed_report /NWAFC_PR_88-21.pdf. - Meinhofer A. The war on drugs: Estimating the effect of prescription drug supply-side interventions (September 7, 2016). 2016. [February 24, 2017]. https://ssrn
.com/abstract=2716974. - Mennis J, Stahler GJ. Racial and ethnic disparities in outpatient substance use disorder treatment episode completion for different substances. Journal of Substance Abuse Treatment. 2016;63:25–33. [PubMed]
- Miaskowski C, Dodd MJ, West C, Paul SM, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: A significant barrier to effective cancer pain management. Journal of Clinical Oncology. 2001;19(23):4275–4279. [PubMed]
- Michna E, Cheng WY, Korves C, Birnbaum H, Andrews R, Zhou Z, Joshi AV, Schaaf D, Mardekian J, Sheng M. Systematic literature review and metaanalysis of the efficacy and safety of prescription opioids, including abuse-deterrent formulations, in non-cancer pain management. Pain Medicine. 2014;15(1):79–92. [PubMed]
- Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of the medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. Journal of Substance Abuse Treatment. 2016;67:9–14. [PubMed]
- Moore RA. What works for whom? Determining the efficacy and harm of treatments for pain. Pain. 2013;154(1):S77–S86. [PubMed]
- Morden NE, Zerzan JT, Rue TC, Heagerty PJ, Roughead EE, Soumerai SB, Ross-Degnan D, Sullivan SD. Medicaid prior authorization and controlled-release oxycodone. Medical Care. 2008;46(6):573–580. [PubMed]
- Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger LA. A review of opioid overdose prevention and naloxone prescribing: Implications for translating community programming into clinical practice. Substance Abuse. 2015;36(2):240–253. [PMC free article] [PubMed]
- Murnion BP, Gnjidic D, Hilmer SN. Prescription and administration of opioids to hospital in-patients, and barriers to effective use. Pain Medicine. 2010;11(1):58–66. [PubMed]
- NAMSDL (National Alliance for Model State Drug Laws). Compilation of prescription monitoring program maps. 2016. [January 18, 2017]. http://www
.namsdl.org /library/CAE654BF-BBEA-211E-694C755E16C2DD21. - NAMSDL. Frequency of prescription drug monitoring program (PMP) data reporting—Map. 2017. [July 10, 2017]. http://www
.namsdl.org /library/D33048BB-E629-981F-648E746714E2A194. - Network for Public Health Law. Legal interventions to reduce overdose mortality: Naloxone access and overdose Good Samaritan laws. 2016. [May 25, 2017]. https://www
.networkforphl .org/_asset/qz5pvn /network-naloxone-10-4.pdf. - Nielsen S, Larney S, Farrell M, Degenhardt L. Community pharmacist knowledge, attitudes and confidence regarding naloxone for overdose reversal. Addiction. 2016;111(12):2177–2186. [PubMed]
- NIH (National Institutes of Health). Centers of Excellence in Pain Education (CoEPEs). 2017. [March 21, 2017]. https:
//painconsortium .nih.gov/nih_pain_programs/coepes.html. - NRC (National Research Council). Informing America's policy on illegal drugs: What we don't know keeps hurting us. Washington, DC: National Academy Press; 2001.
- Oehler EC, Day RL, Robinson DB, Brown LH. Has the rescheduling of hydrocodone changed ED prescribing practices? American Journal of Emergency Medicine. 2016;34(12):2388–2391. [PubMed]
- Back policy changes fact sheet. 2016. [May 22, 2017]. Oregon Health Plan. https://www
.oregon.gov /oha/herc/FactSheet /Back-policy-changes-fact-sheet.pdf. - Paone D, Tuazon E, Kattan J, Nolan ML, O'Brien DB, Dowell D, Farley TA, Kunins HV. Decrease in rate of opioid analgesic overdose deaths—Staten Island, New York City, 2011–2013. Morbidity and Mortality Weekly Report. 2015;64(18):491–494. [PMC free article] [PubMed]
- Parmar MKB, Strang J, Choo L, Meade AM, Bird SM. Randomized controlled pilot trial of naloxone-on-release to prevent post-prison opioid overdose deaths. Addiction. 2017;112(3):502–515. [PMC free article] [PubMed]
- Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Affairs. 2016;35(7):1324–1332. [PMC free article] [PubMed]
- Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Medicine. 2011;12(5):747–754. [PubMed]
- Pergolizzi JV Jr., Raffa RB, LeQuang JA. The Centers for Disease Control and Prevention opioid guidelines: Potential for unintended consequences and will they be abused? Journal of Clinical Pharmacy and Therapeutics. 2016;41(6):592–593. [PubMed]
- Petry NM, Bickel WK. Polydrug abuse in heroin addicts: A behavioral economic analysis. Addiction. 1998;93(3):321–335. [PubMed]
- Pew Charitable Trusts. Curbing prescription drug abuse with patient review and restriction programs: Learning from Medicaid agencies. 2016. [March 21, 2017]. http://www
.pewtrusts .org/~/media/assets/2016 /03/curbing_prescription _drug_abuse_with _patient_review_and _restriction_programs.pdf. - Policy Surveillance Program. Syringe distribution laws. 2012. [April 17, 2017]. http://lawatlas
.org/datasets /syringe-policies-laws-regulating-non-retail-distribution-of-drug-parapherna. - Poncelet A, Bokser S, Calton B, Hauer KE, Kirsch H, Jones T, Lai CJ, Mazotti L, Shore W, Teherani A, Tong L, Wamsley M, Robertson P. Development of a longitudinal integrated clerkship at an academic medical center. Medical Education Online. 2011;16 [PMC free article] [PubMed]
- Qaseem A, Wilt TJ, McLean RM, M.A. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine. 2017;166(7):I20–I21. [PubMed]
- Qureshi N, Wesolowicz LA, Liu CM, Tungol LA. Effectiveness of a retrospective drug utilization review on potentially unsafe opioid and central nervous system combination therapy. Journal of Managed Care and Specialty Pharmacy. 2015;21(10):938–944. [PubMed]
- Rathlev N, Almomen R, Deutsch A, Smithline H, Li H, Visintainer P. Randomized controlled trial of electronic care plan alerts and resource utilization by high frequency emergency department users with opioid use disorder. Western Journal of Emergency Medicine. 2016;17(1):28–34. [PMC free article] [PubMed]
- Reddy A, de la Cruz M, Rodriguez EM, Thames J, Wu J, Chisholm G, Liu D, Frisbee-Hume S, Yennurajalingam S, Hui D, Cantu H, Marin A, Gayle V, Shinn N, Xu A, Williams J, Bruera E. Patterns of storage, use, and disposal of opioids among cancer outpatients. The Oncologist. 2014;19(7):780–785. [PMC free article] [PubMed]
- Rees DI, Sabia JJ, Argys LM, Latshaw J, Dave D. With a little help from my friends: The effects of naloxone access and good Samaritan laws on opioid-related deaths. Cambridge, MA: National Bureau of Economic Research; 2017. [February 23, 2017]. Working Paper 23171. http://www
.nber.org/papers/w23171. - Relman AS. Separating continuing medical education from pharmaceutical marketing. Journal of the American Medical Association. 2001;285(15):2009–2012. [PubMed]
- Reuter P, Kleiman M. Risks and prices: An economic analysis of drug enforcement. In: Tonry M, Morris N, editors. Crime and justice: A review of research. Vol. 7. Chicago, IL: University of Chicago Press; 1986.
- Rocheleau AM, Boyum D. Measuring heroin availability in three cities. Washington, DC: Office of National Drug Control Policy; 1994.
- Rowe C, Santos M, Wheeler E, Vittinghoff E, Davidson P, Coffin PO. Predictors of participant engagement and naloxone utilization in a community-based naloxone distribution program. Addiction. 2015;110(8):1301–1310.[PMC free article] [PubMed]
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. Morbidity and Mortality Weekly Report. 2016;65:1445–1452. [PubMed]
- Rutkow L, Chang HY, Daubreese M, Webster DW, Stuart EA, Alexander GC. Effect of Florida's prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Internal Medicine. 2015;175(10):1642–1649. [PubMed]
- SAMHSA (Substance Abuse and Mental Health Services Administration). Bibliographic support for the Syringe Services Program (SSP). 2011. [January 5, 2017]. http://archive
.samhsa.gov/ssp/ - SAMHSA. A treatment improvement protocol: Managing chronic pain in adults with or in recovery from substance use disorders. 2012. [March 21, 2017]. http://store
.samhsa.gov /shin/content/SMA12-4671/TIP54.pdf. - Samuels E. Emergency department naloxone distribution: A Rhode Island department of health, recovery community, and emergency department partnership to reduce opioid overdose deaths. Rhode Island Medical Journal (2013). 2014;97(10):38–39. [PubMed]
- Sapienza FL. Abuse deterrent formulations and the Controlled Substances Act (CSA). Drug & Alcohol Dependence. 2006;83:S23–S30. [PubMed]
- Satterfield JM, Mitteness LS, Tervalon M, Adler N. Integrating the social and behavioral sciences in an undergraduate medical curriculum: The UCSF essential core. Academic Medicine. 2004;79(1):6–15. [PubMed]
- Schwartz RP, Gryczynski J, O'Grady KE, Sharfstein JM, Warren G, Olsen Y, Mitchell SG, Jaffe JH. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. American Journal of Public Health. 2013;103(5):917–922. [PMC free article] [PubMed]
- Opening a supervised injection facility for people who inject drugs could save millions. ScienceDaily. 2016 December 14; [April 17, 2017]; ScienceDaily. https://www
.sciencedaily .com/releases/2016/12/161214115102 .htm. - Seago S, Hayek A, Pruszynski J, Newman MG. Change in prescription habits after federal rescheduling of hydrocodone combination products. Proceedings (Baylor University. Medical Center); 2016. p. 268. [PMC free article] [PubMed]
- Severtson SG, Bartelson BB, Davis JM, Munoz A, Schneider MF, Chilcoat H, Coplan PM, Surratt H, Dart RC. Reduced abuse, therapeutic errors, and diversion following reformulation of extended-release oxycodone in 2010. The Journal of Pain. 2013;14(10):1122–1130. [PubMed]
- Silverman E. Senators ask drug makers to explain prices for opioid overdose antidote. STAT. 2016 June 7; [November 4, 2016]; https://www
.statnews .com/pharmalot/2016/06 /07/naloxone-opioids-heroin-drug-prices/ - Spiller HA, Scaglione JM, Aleguas A, Foster H, Durback-Morris L, Scharman EJ, Baker SD. Effect of scheduling tramadol as a controlled substance on poison center exposures to tramadol. Annals of Pharmacotherapy. 2010;44(6):1016–1021. [PubMed]
- Stein MD, Anderson BJ, Thurmond P, Bailey GL. Comparing the life concerns of prescription opioid and heroin users. Journal of Substance Abuse Treatment. 2015;48(1):43–48. [PMC free article] [PubMed]
- Stewart H, Malinowski A, Ochs L, Jaramillo J, McCall K, Sullivan M. Inside Maine's medicine cabinet: Findings from the Drug Enforcement Administration's medication take-back events. American Journal of Public Health. 2015;105(1):e65–e71. [PMC free article] [PubMed]
- Stogner J, Khey DN, Griffin OH, Miller BL, Boman JH. Regulating a novel drug: An evaluation of changes in use of Salvia divinorum in the first year of Florida's ban. International Journal of Drug Policy. 2012;23(6):512–521. [PubMed]
- Strang J, Powis B, Best D, Vingoe L, Griffiths P, Taylor C, Welch S, Gossop M. Preventing opiate overdose fatalities with take-home naloxone: Pre-launch study of possible impact and acceptability. Addiction. 1999;94(2):199–204. [PubMed]
- Sullivan MD, Bauer AM, Fulton-Kehoe D, Garg RG, Turner JA, Wickizer T, Franklin GM. Trends in opioid dosing among Washington State Medicaid patients before and after opioid dosing guideline implementation. The Journal of Pain. 2016;17(5):561–568. [PubMed]
- Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Internal Medicine. 2016;176(9):1286–1293. [PubMed]
- Surratt HL, O'Grady C, Kurtz SP, Stivers Y, Cicero TJ, Dart RC, Chen M. Reductions in prescription opioid diversion following recent legislative interventions in Florida. Pharmacoepidemiology and Drug Safety. 2014;23(3):314–320. [PubMed]
- Terrab M, Odoni AR. Strategic flow management for air traffic control. Operations Research. 1993;41(1):138–152.
- Thomas K. Doubts raised about off-label use of Subsys, a strong painkiller. The New York Times. 2014a May 13; [January 5, 2017]; https://www
.nytimes.com /2014/05/14/business /doubts-raised-about-off-label-use-of-subsys-a-strong-painkiller.html. - Thomas K. Using doctors with troubled pasts to market a painkiller. The New York Times. 2014b November 27; [January 5, 2017]; https://www
.nytimes.com /2014/11/28/business /drug-maker-gave-large-payments-to-doctors-with-troubled-track-records.html. - Thomas K. Nurse pleads guilty to taking kickbacks from drug maker. The New York Times. 2015 June 25; [January 5, 2017]; https://www
.nytimes.com /2015/06/26/business /nurse-pleads-guilty-to-taking-kickbacks-from-drug-maker .html. - Thomas K. Drug maker's former employees accused of shady dealings with doctors. The New York Times. 2016a June 10; [January 5, 2017]; https://www
.nytimes.com /2016/06/11/business /drug-makers-former-employees-accused-of-shady-dealings-with-doctors.html. - Thomas K. Former Insys officials charged in scheme to push its painkiller. The New York Times. 2016b December 8; [January 5, 2017]; https://www
.nytimes.com /2016/12/08/business /insys-therapeutics-arrests-fentanyl .html. - Trafton J, Martins S, Michel M, Lewis E, Wang D, Combs A, Scates N, Tu S, Goldstein MK. Evaluation of the acceptability and usability of a decision support system to encourage safe and effective use of opioid therapy for chronic, noncancer pain by primary care providers. Pain Medicine. 2010;11:575–585. [PubMed]
- Traynor K. Maine enacts statewide limits on opioid prescribing. American Journal of Health- System Pharmacy. 2016;73(12):854–856. [PubMed]
- Turk D, Burwinkle T. Clinical outcomes, cost-effectiveness, and the role of psychology in treatments for chronic pain sufferers. Professional Psychology: Research and Practice. 2005;36(6):602–610.
- UCSF (University of California, San Francisco). Biopsychosocial and cultural issues in action: Mr. Danovic outpatient case and review. 2017. [April 26, 2017]. http://www
.osher.ucsf .edu/education/integrative-medicine-curriculum /required-curriculum/#Danovic. - VA (U.S. Department of Veterans Affairs). Recommendations for issuing naloxone rescue for the VA Opioid Overdose Education and Naloxone Distribution (OEND) program. 2016. [December 5, 2016]. https://www
.pbm.va.gov /PBM/clinicalguidance /clinicalrecommendations /Naloxone_HCl _Rescue_Kits_Recommendations_for_Use.pdf. - VA and DoD (U.S. Department of Defense). VA/DoD Clinical Practice Guideline for Opioid Therapy for Chronic Pain. 2017. [August 16, 2017]. https://www
.healthquality .va.gov/guidelines /Pain/cot/VADoDOTCPGProviderSummary022817.pdf. - Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. New England Journal of Medicine. 2014;370(22):2063–2066. [PubMed]
- Wakeland W, Nielsen A, Geissert P. Dynamic model of nonmedical opioid use trajectories and potential policy interventions. The American Journal of Drug and Alcohol Abuse. 2015;41(6):508–518. [PMC free article] [PubMed]
- Walgreens. Walgreens expands availability of naloxone without a prescription to 33 states and Washington D.C.2016. [April 17, 2017]. Press Release. http://news
.walgreens .com/press-releases /general-news/walgreens-expands-availability-of-naloxone-without-a-prescription-to-33-states-and-washington-dc.htm. - Walgreens. Walgreens medication disposal program collects 72 tons of unused medications in first year; opioid antidote medication naloxone available without prescription at Walgreens in 44 states. 2017. [May 5, 2017]. Press Release. http://news
.walgreens .com/press-releases /general-news/walgreens-medication-disposal-program-collects-72-tons-of-unused-medications-in-first-year-opioid-antidote-medication-naloxone-available-without-prescription-at-walgreens-in-44-states .htm. - Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: Interrupted time series analysis. British Medical Journal. 2013;346:f174. [PMC free article] [PubMed]
- Wang V. CVS pays $3.5m to settle claims it filled fake painkiller prescriptions. 2016. [March 21, 2017].https://www
.bostonglobe .com/metro/2016/06 /30/cvs-pays-million-settle-federal-probe-that-found-pharmacists-filled-forged-prescriptions /btKqNm4tYmglO3s8qm8V3I /story.html. - Supervised injection facilities. 2017. [April 17, 2017]. We are the Drug Policy Alliance. http://www
.drugpolicy .org/supervised-injection-facilities. - Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127–2132. [PubMed]
- Welham GC, Mount JK, Gilson AM. Type and frequency of opioid pain medications returned for disposal. Drugs-Real World Outcomes. 2015;2(2):129–135. [PMC free article] [PubMed]
- Wen H, Schackman BR, Aden B, Bao Y. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to Medicaid enrollees. Health Affairs. 2017;36(4):733–741. [PMC free article] [PubMed]
- WHO (World Health Organization). Cancer pain relief. Geneva, Switzerland: WHO; 1986.
- WSAMDG (Washington State Agency Medical Directors' Group). Interagency guideline on prescribing opioids for pain. 2015. [February 25, 2017]. http://www
.agencymeddirectors .wa.gov/Files /2015AMDGOpioidGuideline.pdf. - WSIN (Western States Information Network). Drug price and purity guide, 2016. Sacramento, CA: WSIN; 2016.
- Wu LT, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence. 2016;169:117–127. [PMC free article] [PubMed]
- Yanovitsky I. The American medicine chest challenge: Evaluation of a drug take-back and disposal campaign. Journal of Studies on Alcohol and Drugs. 2016;77(4):549–555. [PubMed]
- Zeppetella G, Davies AN. Opioids for the management of breakthrough pain in cancer patients. Cochrane Database of Systematic Reviews. 2013;10 CD004311. [PubMed]
- Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, Simoni-Wastila L, Sullivan SD. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Medical Care. 2006;44(11):1005–1010. [PubMed]
Footnotes
- 1
- Vigilance will also be needed to reduce the risk of similar problems in the future with other classes of medications for which there exists demand for clinical uses other than the indicated conditions and/or active black markets for their resale.
- 2
- Of course, even RCTs are not perfect. For example, they may overlook indirect effects on people other than those participating in the study. Parmar and colleagues (2017) describe an RCT of the distribution of naloxone to heroin injectors being released from prison in which only one-third of the naloxone administrations in the treatment group were to the ex-prisoners in the study themselves; the majority of the administrations were to others who were outside the scope of data collection. The trial was closed prematurely as a result of this and related problems.
- 3
- More sophisticated models will have a second pool consisting of people who have temporarily ceased use but are vulnerable to relapse.
- 4
- Severston and colleagues (2013) describe prices that are 22 percent lower. RADARS System Technical Report, 2014-Q2 describes declines closer to 33 percent.
- 5
- Enforcement and punishment strategies for curtailing illegal production and distribution of unapproved/illegal drugs (i.e., heroin and other Schedule I drugs and illegally manufactured versions of legally available drugs) lies outside the scope of this study. However, see the National Research Council report Informing America's Policy on Illegal Drugs: What We Don't Know Keeps Hurting Us (NRC, 2001).
- 6
- This section addresses restrictions on drugs that have been approved by appropriate authorities for medical use, i.e., that are not allowed for nonmedical use. Different policy challenges arise in the design and implementation of regulatory schemes that control access to and use of a drug for nonmedical purposes. Prominent examples are alcohol and marijuana. It is possible to have separate legal regimes for medical and nonmedical uses. All of these issues are beyond the scope of this report.
- 7
- 21 C.F.R. Part 1308.
- 8
- The actions of organized criminal groups also apply here, but they generally are not involved in prescribing.
- 9
- There can be some tension between these objectives. While both interests agree that the first-best outcome is for unused medications to be returned to pharmacies or other institutions that can dispose of them properly, that is the exception, not the norm, and there can be disagreement about what is the best fallback. Some who are concerned about misuse urge that leftover drugs be flushed down the toilet, but that is arguably the worst option from an environmental perspective because sewage treatment plants seldom remove medications from water, and those concerned about environmental consequences may prefer that leftover drugs be disposed of in the trash (Daughton, 2007).
- 10
- Massachusetts Public Law H.4056.
- 11
- The ADF of OxyContin ER also emerged around this time, but this was a national not a state-specific intervention and so cannot account for the peculiar trajectory of outcomes in Florida.
- 12
- See http://painmeded
.com /wp-content/uploads/adobe _captivate_uploads /EdnaUpdate081616/multiscreen.html (accessed August 16, 2017). - 13
- See http://www
.health.utah .gov/prescription/guidelines.html (accessed April 17, 2017). - 14
- Several counties and other localities within Missouri have established their own PDMPs.
- 15
- As of May 2016, these states included Alabama, Alaska, Georgia, Illinois, Indiana, Iowa, Kansas, Minnesota, North Dakota, Oregon, South Carolina, South Dakota, Wisconsin, and Wyoming (NAMSDL, 2016).
- 16
- A logical complement to all patient and public education efforts is a substantial effort to counteract and possibly restrict direct-to-consumer advertising and other promotional efforts by pharmaceutical manufacturers aimed at increasing the use of opioids. This topic is addressed in Chapter 6.
- 17
- Drug Abuse Treatment Act of 2000.
- 18
- See http://www
.temple.edu /lawschool/phrhcs/otc.htm (accessed April 17, 2017). - 19
- See http://leginfo
.legislature .ca.gov/faces/billNavClient .xhtml?bill_id =201320140AB1743&search _keywords= (accessed April 17, 2017). - 20
- See https://www
.manchesternh .gov/Departments/Fire/Safe-Station (accessed April 17, 2017).
- Evidence on Strategies for Addressing the Opioid Epidemic - Pain Management and ...
- Acupuncture for Detoxification in Treatment of Opioid Addiction.PubMed
- Effectiveness of Mindfulness-Based Group Therapy Compared to the Usual Opioid De...
- Introduction - Pain Management and the Opioid Epidemic
External link. Please review our privacy policy.
National Center for Biotechnology Information, U.S. National Library of Medicine8600 Rockville Pike, Bethesda MD, 20894 USA
Policies and Guidelines | Contact
No comments:
Post a Comment